Calyculin A
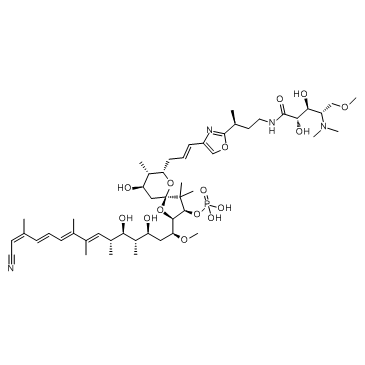
Calyculin A structure
|
Common Name | Calyculin A | ||
|---|---|---|---|---|
| CAS Number | 101932-71-2 | Molecular Weight | 1009.17000 | |
| Density | 1.25g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C50H81N4O15P | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Calyculin ACalyculin A is a potent and cell-permeable protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) inhibitor with IC50s of 0.5 to 1 nM. |
| Name | Calyculin A from Discodermia calyx |
|---|---|
| Synonym | More Synonyms |
| Description | Calyculin A is a potent and cell-permeable protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) inhibitor with IC50s of 0.5 to 1 nM. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.25g/cm3 |
|---|---|
| Molecular Formula | C50H81N4O15P |
| Molecular Weight | 1009.17000 |
| Exact Mass | 1008.54000 |
| PSA | 296.80000 |
| LogP | 5.07988 |
| Index of Refraction | 1.57 |
| Storage condition | 2-8℃ |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H311 + H331-H315 |
| Precautionary Statements | P261-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | 23/24/25-38 |
| Safety Phrases | 36/37/39-45 |
| RIDADR | UN 3462 6.1/PG 3 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
|
A novel receptor cross-talk between the ATP receptor P2Y2 and formyl peptide receptors reactivates desensitized neutrophils to produce superoxide
Exp. Cell Res. 323(1) , 209-17, (2014) Neutrophils express several G-protein coupled receptors (GPCRs) and they cross regulate each other. We described a novel cross-talk mechanism in neutrophils, by which signals generated by the receptor... |
|
|
Detachment-induced autophagy in three-dimensional epithelial cell cultures.
Meth. Enzymol. 452 , 423-39, (2009) Integrin-mediated cell adhesion to extracellular matrix (ECM) is critical for normal epithelial cell survival; cells deprived of ECM contact rapidly undergo apoptotic cell death, termed anoikis. Recen... |
|
|
Unique features of the okadaic acid activity class of tumor promoters.
J. Cancer Res. Clin. Oncol. 125 , 150-155, (1999) Following the first report of tumor promotion by okadaic acid in 1988, we further identified additional tumor promoters of the okadaic acid activity class, such as calyculin A and microcystin-LR. Howe... |
| Calyculin A,N-[(3S)-[4-(1E)-3-[(2R,3R,4R,7S,8S,9R)-2-[(1S,3S,4S,5R,7E,9E,11E,13Z)-14-Cyano-3,5-dihydroxy-1-methoxy-4,6,8,9,13-pentamethyl-7,9,11,13-tetradecatetraenyl]-9-hydroxy-4,4,8-trimethyl-3-(phosphonooxy)-1,6-dioxaspiro[4.5]dec-7-yl]-1-propenyl]-2-o |
| MFCD00133156 |
| Calyculin A |