GSK2126458(GSK458)
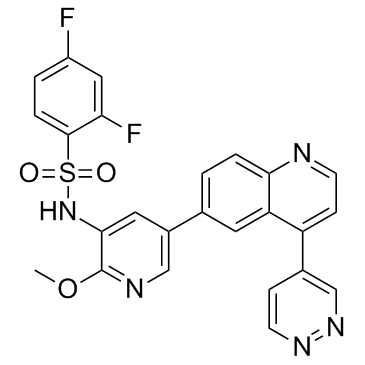
GSK2126458(GSK458) structure
|
Common Name | GSK2126458(GSK458) | ||
|---|---|---|---|---|
| CAS Number | 1086062-66-9 | Molecular Weight | 505.496 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 715.6±70.0 °C at 760 mmHg | |
| Molecular Formula | C25H17F2N5O3S | Melting Point | 187-189℃ | |
| MSDS | N/A | Flash Point | 386.6±35.7 °C | |
Use of GSK2126458(GSK458)Omipalisib (GSK2126458) is a highly selective and potent inhibitor of PI3K with Kis of 0.019 nM/0.13 nM/0.024 nM/0.06 nM and 0.18 nM/0.3 nM for p110α/β/δ/γ, mTORC1/2, respectively. |
| Name | 2,4-difluoro-N-[2-methoxy-5-(4-pyridazin-4-ylquinolin-6-yl)pyridin-3-yl]benzenesulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | Omipalisib (GSK2126458) is a highly selective and potent inhibitor of PI3K with Kis of 0.019 nM/0.13 nM/0.024 nM/0.06 nM and 0.18 nM/0.3 nM for p110α/β/δ/γ, mTORC1/2, respectively. |
|---|---|
| Related Catalog | |
| Target |
p110α:0.019 nM (Ki) p110β:0.13 nM (Ki) p110δ:0.024 nM (Ki) p110γ:0.06 nM (Ki) p110α-H1047R:0.009 nM (Ki) p110α-E545K:0.008 nM (Ki) p110α-E542K:0.008 nM (Ki) mTORC1:0.18 nM (Ki) mTORC2:0.3 nM (Ki) |
| In Vitro | Omipalisib (GSK2126458) potently inhibits the activity of common activating mutants of p110α (E542K, E545K, and H1047R) found in human cancer with Ki of 8 pM, 8 pM and 9 pM, respectively. Omipalisib causes a significant reduction in the levels of pAkt-S473 with remarkable potency in T47D and BT474 cells with IC50 of 0.41 nM and 0.18 nM, respectively. Furthermore, Omipalisib (GSK2126458) leads to a G1 cell cycle arrest and produces the inhibitory effect on cell proliferation in a large panel of cell lines, including T47D and BT474 breast cancer lines with IC50 of 3 nM and 2.4 nM, respectively[1]. The combination of Omipalisib or GSK1120212 with Omipalisib enhances cell growth inhibition and decreases S6 ribosomal protein phosphorylation in drug-resistant clones from the A375 BRAF(V600E) and the YUSIT1 BRAF(V600K) melanoma cell lines[2]. Omipalisib (GSK2126458) potentiates the antiproliferative activity of DDR1-IN-1 in colorectal cancer cell lines[3]. |
| In Vivo | In a BT474 human tumor xenograft model, Omipalisib (GSK2126458) treatment results in a dose-dependent reduction in pAkt-S473 levels, and exhibits dose-dependent tumor growth inhibition at a low dose of 300 μg/kg. Besides, Omipalisib (GSK2126458) shows low blood clearance and good oral bioavailability in four preclinical species (mouse, rat, dog, and monkey)[1]. |
| Cell Assay | BT474, HCC1954 and T-47D (human breast) are cultured in RPMI-1640 containing 10% fetal bovine serum at 37°C in 5% CO2 incubator. Cells are split into T75 flask two to three days prior to assay set up at density which yields approximately 70-80% confluence at time of harvest for assay. Cells are harvested using 0.25% trypsin-EDTA. Cell counts are performed on cell suspension using Trypan Blue exclusion staining. Cells are then plated in 384 well black flat bottom polystyrene in 48 μL of culture media per well at 1,000 cells/well. All plates are placed at 5% CO2, 37°C overnight and Omipalisib (GSK2126458) is added the following day. One plate is treated with CellTiter-Glo for a day 0 (t=0) measurement and read as described below. Omipalisib (GSK2126458) is prepared in clear bottom polypropylene 384 well plates with consecutive two fold dilutions. 4 μL of these dilutions are added to 105 μL culture media, after mixing the solution, 2 μL of these dilutions are added into each well of the cell plates. The final concentration of DMSO in all wells is 0.15%. Cells are incubated at 37°C, 5% CO2 for 72 hours. Following 72 hours of incubation with Omipalisib each plate is developed and read. CellTiter-Glo reagent is added to assay plates using a volume equivalent to the cell culture volume in the wells. Plates are shaken for approximately two minutes and incubated at room temperature for approximately 30 minutes and chemiluminescent signal is read on the Analyst GT reader. Results are expressed as a percent of the t=0 and plotted against the Omipalisib (GSK2126458) concentration. Cell growth inhibition is determined for Omipalisib (GSK2126458) by fitting the dose response with a 4 or 6 parameter curve fit using XLfit software and determining the concentration that inhibits 50% of the cell growth (gIC50) with the Y min as the t=0 and Y max as the DMSO control. Value from wells with no cells is subtracted from all samples for background correction.. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 715.6±70.0 °C at 760 mmHg |
| Melting Point | 187-189℃ |
| Molecular Formula | C25H17F2N5O3S |
| Molecular Weight | 505.496 |
| Flash Point | 386.6±35.7 °C |
| Exact Mass | 505.102020 |
| PSA | 115.34000 |
| LogP | 3.81 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.660 |
| Storage condition | -20℃ |
| Hazard Codes | Xi |
|---|
| Precursor 10 | |
|---|---|
| DownStream 0 | |
| 2,4-difluoro-N-(2-methoxy-5-(4-(pyridazin-4-yl)quinolin-6-yl)pyridin-3-yl)benzenesulfonamide |
| 2,4-Difluoro-N-{2-methoxy-5-[4-(4-pyridazinyl)-6-quinolinyl]-3-pyridinyl}benzenesulfonamide |
| GSK2126458 |
| Omipalisib |
| gsk2126458 (gsk458) |
| 2,4-difluoro-N-[2-methoxy-5-(4-pyridazin-4-ylquinolin-6-yl)pyridin-3-yl]benzenesulfonamide |
| GSK 2126458 |
| gsk-212 |
| Benzenesulfonamide, 2,4-difluoro-N-[2-methoxy-5-[4-(4-pyridazinyl)-6-quinolinyl]-3-pyridinyl]- |
| GSK458 |
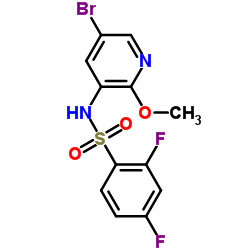 CAS#:1086063-46-8
CAS#:1086063-46-8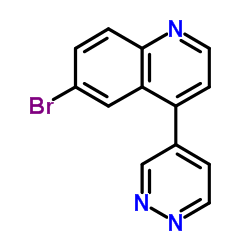 CAS#:1086063-18-4
CAS#:1086063-18-4 CAS#:884495-39-0
CAS#:884495-39-0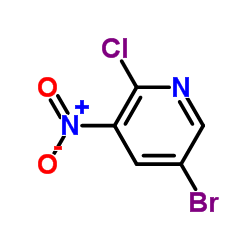 CAS#:67443-38-3
CAS#:67443-38-3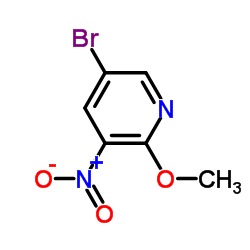 CAS#:152684-30-5
CAS#:152684-30-5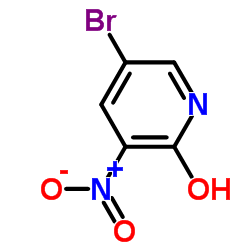 CAS#:15862-34-7
CAS#:15862-34-7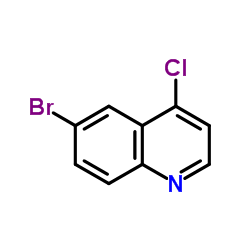 CAS#:65340-70-7
CAS#:65340-70-7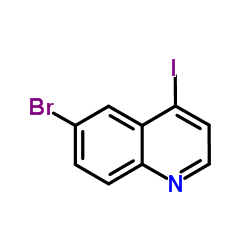 CAS#:927801-23-8
CAS#:927801-23-8 CAS#:1086062-75-0
CAS#:1086062-75-0 CAS#:145369-94-4
CAS#:145369-94-4