Paroxetine hydrochloride hydrate
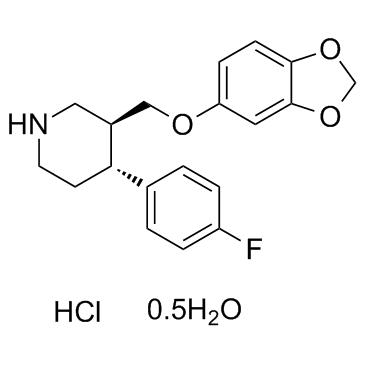
Paroxetine hydrochloride hydrate structure
|
Common Name | Paroxetine hydrochloride hydrate | ||
|---|---|---|---|---|
| CAS Number | 110429-35-1 | Molecular Weight | 374.83 | |
| Density | 1.213g/cm3 | Boiling Point | 451.7ºC at 760mmHg | |
| Molecular Formula | C19H22ClFNO3.5 | Melting Point | 121-131ºC | |
| MSDS | Chinese USA | Flash Point | 227ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Paroxetine hydrochloride hydrateParoxetine hydrochloride hemihydrate is a potent selective serotonin-reuptake inhibitor, commonly prescribed as an antidepressant and has GRK2 inhibitory ability with IC50 of 14 μM. |
| Name | Paroxetine hydrochloride hemihydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Paroxetine hydrochloride hemihydrate is a potent selective serotonin-reuptake inhibitor, commonly prescribed as an antidepressant and has GRK2 inhibitory ability with IC50 of 14 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 14 μM (GRK2)[3] |
| In Vitro | Paroxetine (1 μM and 10 μM) distinctly restrains T cell migration induced by CX3CL1 through inhibiting GRK2. Paroxetine inhibits GRK2 induced activation of ERK[1]. Paroxetine (10 μM) reduces pro-inflammatory cytokines in LPS-stimulated BV2 cells. Paroxetine (0-5 μM) leads to a dose-dependent inhibition on LPS-induced production of TNF-α and IL-1β in BV2 cells. Paroxetine also inhibits lipopolysaccharide (LPS)-induced nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression in BV2 cells. Paroxetine (5 μM) blocks LPS-induced JNK activation and attenuates baseline ERK1/2 activity in BV2 cells. Paroxetine relieves microglia-mediated neurotoxicity, and suppresses LPS-stimulated pro-inflammatory cytokines and NO in primary microglial cells[4]. |
| In Vivo | Paroxetine treatment obviously attenuates the symptoms of CIA rats. Paroxetine treatment clearly prevents the histological damage of joints and alleviates T cells infiltration into synovial tissue. Paroxetine reveals a strong effect on inhibiting CX3CL1 production in synovial tissues[1]. Paroxetine (20 mg/kg/day) reduces the myocyte cross-sectional area in rat and ROS formation in the remote myocardium. Paroxetine reduces the susceptibility to ventricular tachycardia. Paroxetine treatment following MI decreases LV remodeling and susceptibility to arrhythmias, probably by reducing ROS formation[2]. In CCI paroxetine-treated group, paroxetine (10 mg/kg, i.p.) produces hyperalgesia at days 7 and 10 (P<0.01), but a decrease in pain behavior is seen at day 14. Moreover, paroxetine (10 mg/kg) significantly attenuates tactile hypersensitivity when compared to CCI vehicle-treated group[5]. |
| Cell Assay | Cell viability is determined by the tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. BV2 and primary microglial cells are initially seeded into 96-well plates at a density of 1×104 cells/well and 5×104 cells/well, respectively. Following treatment, MTT (5 mg/mL in PBS) is added to each well and incubated at 37°C for four hours. The resulting formazan crystals are dissolved in dimethylsulfoxide (DMSO). The optical density is measured at 570 nm, and results are expressed as a percentage of surviving cells compared with the control. |
| Animal Admin | Animals are divided into two main groups: 1) pre-emptive and 2) post-injury group. Each main group is divided into three different subgroups: I) CCI vehicle-treated group, II) sham group, and III) CCI paroxetine-treated group. Vehicle is injected i.p. to CCI and sham-operated animals. In the pre-emptive study, paroxetine (10 mg/kg) is injected 1 h before surgery and continued daily until day 14 post surgery. In the post-injury group, paroxetine (10 mg/kg) is administered at day 7 post injury and continued daily until day 14. All behavioral tests are recorded on day 0 (control day) before surgery and on days 1, 3, 5, 7, 10, and 14 post-nerve injury. |
| References |
| Density | 1.213g/cm3 |
|---|---|
| Boiling Point | 451.7ºC at 760mmHg |
| Melting Point | 121-131ºC |
| Molecular Formula | C19H22ClFNO3.5 |
| Molecular Weight | 374.83 |
| Flash Point | 227ºC |
| PSA | 39.72000 |
| LogP | 4.45730 |
| Appearance of Characters | white |
| InChIKey | QRQSGFFISBKLMZ-YHOFXEKLSA-N |
| SMILES | Cl.Fc1ccc(C2CCNCC2COc2ccc3c(c2)OCO3)cc1.O |
| Water Solubility | Slightly soluble in water, freely soluble in methanol, sparingly soluble in ethanol (96 per cent) and in methylene chloride. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P301 + P312 + P330-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | F,C |
| Risk Phrases | 11-34-36/37/38-22 |
| Safety Phrases | 16-26-36/37/39-45-36 |
| RIDADR | 3077.0 |
| RTECS | TM4569320 |
| HS Code | 2934999090 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
A new SiF-Dipropargyl glycerol scaffold as a versatile prosthetic group to design dimeric radioligands: synthesis of the [(18) F]BMPPSiF tracer to image serotonin receptors.
ChemMedChem 9(2) , 337-49, (2014) A novel SiX-dipropargyl glycerol scaffold (X: H, F, or (18) F) was developed as a versatile prosthetic group that provides technical advantages for the preparation of dimeric radioligands based on sil... |
|
|
A thorough QTc study of 3 doses of iloperidone including metabolic inhibition via CYP2D6 and/or CYP3A4 and a comparison to quetiapine and ziprasidone.
J. Clin. Psychopharmacol. 33(1) , 3-10, (2013) The potential for iloperidone, a D2/5-HT2A antipsychotic, to affect the heart rate-corrected QT interval (QTc) was assessed in the absence and presence of metabolic inhibitors in a randomized, open-la... |
|
|
A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity.
Sci. Transl. Med. 7 , 290ra89, (2015) Currently, no approved therapeutics exist to treat or prevent infections induced by Ebola viruses, and recent events have demonstrated an urgent need for rapid discovery of new treatments. Repurposing... |
| paroxetine hydrochloride ;(-)trans-3-[(1,3-benzodioxol-5-yloxy) methyl]-4-(4-fluorophenyl) paroxetine hydrochloride |
| (3S,4R)-3-[(1,3-Benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine hydrochloride hydrate (2:2:1) |
| Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, (3S,4R)-, hydrochloride, hydrate (2:2:1) |
| Paroxetine Hydrochloride HeMihydrate |
| Paroxetine hydrochl |
| Paroxetine hydrochloride |
| Paroxetine hydrochloride hydrate |
| Paroxetine (hydrochloride hemihydrate) |
![phenyl (3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine-1-carboxylate Structure](https://image.chemsrc.com/caspic/089/253768-88-6.png) CAS#:253768-88-6
CAS#:253768-88-6![(3S,4R)-trans-1-benzyloxycarbonyl-4-(4-fluorophenyl)-3-[(3,4-methylenedioxyphenyl)oxymethyl]piperidine Structure](https://image.chemsrc.com/caspic/428/246850-71-5.png) CAS#:246850-71-5
CAS#:246850-71-5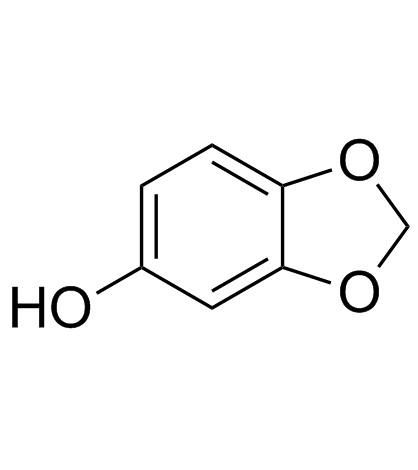 CAS#:533-31-3
CAS#:533-31-3 CAS#:61869-08-7
CAS#:61869-08-7 CAS#:459-57-4
CAS#:459-57-4 CAS#:200572-33-4
CAS#:200572-33-4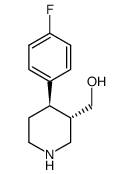 CAS#:125224-43-3
CAS#:125224-43-3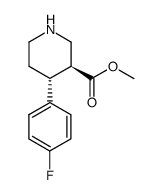 CAS#:872544-47-3
CAS#:872544-47-3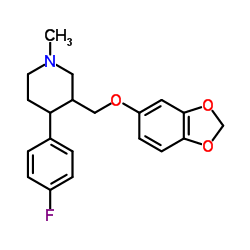 CAS#:110429-36-2
CAS#:110429-36-2