BYK204165
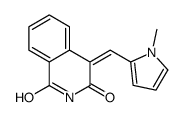
BYK204165 structure
|
Common Name | BYK204165 | ||
|---|---|---|---|---|
| CAS Number | 1104546-89-5 | Molecular Weight | 252.26800 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H12N2O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of BYK204165BYK204165 is a potent PARP inhibitor. BYK204165 inhibits cell-free recombinant human PARP-1 (hPARP-1) with a pIC50 of 7.35 (pKi=7.05), and murine PARP-2 (mPARP-2) with a pIC50 of 5.38, respectively. BYK204165 displays 100-fold selectivity for PARP-1[1]. |
| Name | (4Z)-4-[(1-Methyl-1H-pyrrol-2-yl)methylene]-1,3(2H,4H)-isoquinoli nedione |
|---|---|
| Synonym | More Synonyms |
| Description | BYK204165 is a potent PARP inhibitor. BYK204165 inhibits cell-free recombinant human PARP-1 (hPARP-1) with a pIC50 of 7.35 (pKi=7.05), and murine PARP-2 (mPARP-2) with a pIC50 of 5.38, respectively. BYK204165 displays 100-fold selectivity for PARP-1[1]. |
|---|---|
| Related Catalog | |
| Target |
hPARP-1:7.35 (pIC50) mPARP-2:5.38 (pIC50) |
| In Vitro | In kinetic experiments with human PARP-1, BYK204165 exhibits potent and competitive inhibition of enzyme activity, yielding a pKi value of 7.05[1]. BYK204165 exhibits low potency of PARP inhibition in C4I cells (pIC50 of 5.75)[1]. |
| In Vivo | BYK204165 is not investigated in vivo because of its short half-time (t1/2) of 23 min measured at rat microsomes in vitro[1]. |
| References |
| Molecular Formula | C15H12N2O2 |
|---|---|
| Molecular Weight | 252.26800 |
| Exact Mass | 252.09000 |
| PSA | 54.59000 |
| LogP | 1.84610 |
|
Alternative NHEJ Pathway Components Are Therapeutic Targets in High-Risk Neuroblastoma.
Mol. Cancer Res. 13(3) , 470-82, (2015) In neuroblastoma, MYCN genomic amplification and segmental chromosomal alterations including 1p or 11q loss of heterozygocity and/or 17q gain are associated with progression and poor clinical outcome.... |
| 4-(1-methyl-1H-pyrrol-2-ylmethylene)-4H-isoquinolin-1,3-dione |