Tris(perfluorophenyl)borane
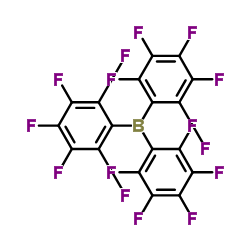
Tris(perfluorophenyl)borane structure
|
Common Name | Tris(perfluorophenyl)borane | ||
|---|---|---|---|---|
| CAS Number | 1109-15-5 | Molecular Weight | 511.98 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 327.3±42.0 °C at 760 mmHg | |
| Molecular Formula | C18BF15 | Melting Point | 126-131 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 151.7±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Tris(perfluorophenyl)boraneTris(perfluorophenyl)borane is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Tris(pentafluorophenyl)borane |
|---|---|
| Synonym | More Synonyms |
| Description | Tris(perfluorophenyl)borane is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 327.3±42.0 °C at 760 mmHg |
| Melting Point | 126-131 °C(lit.) |
| Molecular Formula | C18BF15 |
| Molecular Weight | 511.98 |
| Flash Point | 151.7±27.9 °C |
| Exact Mass | 511.985352 |
| LogP | 5.91 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.455 |
| InChIKey | OBAJXDYVZBHCGT-UHFFFAOYSA-N |
| SMILES | Fc1c(F)c(F)c(B(c2c(F)c(F)c(F)c(F)c2F)c2c(F)c(F)c(F)c(F)c2F)c(F)c1F |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25;R36/37/38 |
| Safety Phrases | S60-S61-S62-S45-S37/39-S26-S36 |
| RIDADR | UN 1268 3/PG 2 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2931900090 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
Reactivity of Amine/E(C6F5)3 (E = B, Al) Lewis Pairs toward Linear and Cyclic Acrylic Monomers: Hydrogenation vs. Polymerization.
Molecules 20 , 9575-90, (2015) This work reveals the contrasting reactivity of amine/E(C6F5)3 (E = B, Al) Lewis pairs toward linear and cyclic acrylic monomers, methyl methacrylate (MMA) and biorenewable γ-methyl-α-methylene-γ-buty... |
|
|
Mussel-Inspired Anchoring of Polymer Loops That Provide Superior Surface Lubrication and Antifouling Properties.
ACS Nano 10 , 930-7, (2016) We describe robustly anchored triblock copolymers that adopt loop conformations on surfaces and endow them with unprecedented lubricating and antifouling properties. The triblocks have two end blocks ... |
|
|
Octahedral Group 4 Metal Complexes That Contain Amine, Amido, and Aminopyridinato Ligands: Synthesis, Structure, and Application in alpha-Olefin Oligo- and Polymerization.
Inorg. Chem. 35 , 6742, (1996) The reaction of trimethylsilyl-substituted 2-aminopyridines with mixed chloro(dialkylamido)metal complexes (titanium and zirconium) leads via amine elimination to octahedral group 4 metal complexes th... |
| tris(perfluorophenyl)borane |
| Perfluorotriphenylboron |
| MFCD00269813 |
| Tris(2,3,4,5,6-pentafluorophenyl)borane |
| Perfluorotriphenylborane |
| Tris(perfluorophenyl)boron |
| Borane, tris(2,3,4,5,6-pentafluorophenyl)- |
| TRIS(PENTAFLUOROPHENYL)BORON |
| tris-(Pentafluorophenyl)borane |
| Tris(pentafluorophenyl)borane |
 CAS#:344-04-7
CAS#:344-04-7 CAS#:363-72-4
CAS#:363-72-4 CAS#:121-43-7
CAS#:121-43-7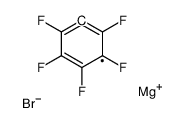 CAS#:879-05-0
CAS#:879-05-0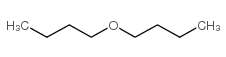 CAS#:142-96-1
CAS#:142-96-1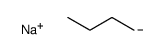 CAS#:3525-44-8
CAS#:3525-44-8 CAS#:1076-44-4
CAS#:1076-44-4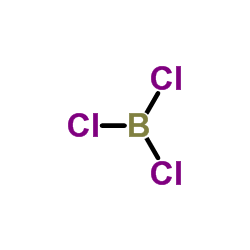 CAS#:10294-34-5
CAS#:10294-34-5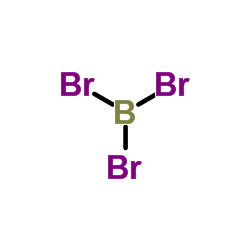 CAS#:10294-33-4
CAS#:10294-33-4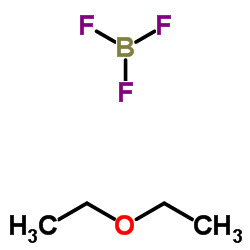 CAS#:109-63-7
CAS#:109-63-7 CAS#:827-15-6
CAS#:827-15-6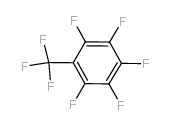 CAS#:434-64-0
CAS#:434-64-0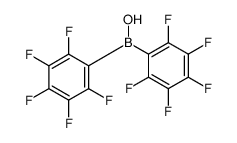 CAS#:2118-02-7
CAS#:2118-02-7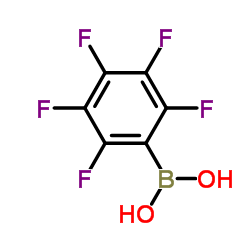 CAS#:1582-24-7
CAS#:1582-24-7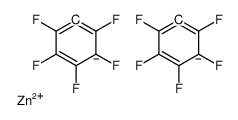 CAS#:1799-90-2
CAS#:1799-90-2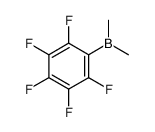 CAS#:363596-54-7
CAS#:363596-54-7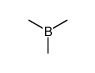 CAS#:593-90-8
CAS#:593-90-8 CAS#:170151-48-1
CAS#:170151-48-1 CAS#:204930-04-1
CAS#:204930-04-1
