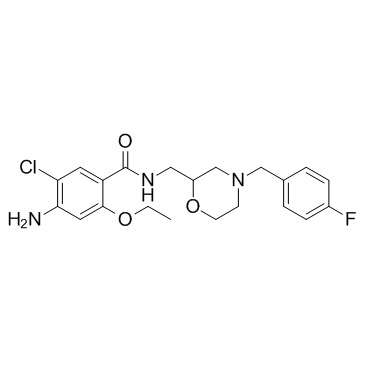
Mosapride citrate
Mosapride citrate structure
|
Common Name | Mosapride citrate | ||
|---|---|---|---|---|
| CAS Number | 112885-42-4 | Molecular Weight | 614.016 | |
| Density | N/A | Boiling Point | 549.2ºC at 760mmHg | |
| Molecular Formula | C27H33ClFN3O10 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 286ºC | |
Use of Mosapride citrateMosapride citrate is a gastroprokinetic agent that acts as a selective 5HT4 agonist.Target: 5HT4 Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastro-oesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).In addition to its prokinetic properties, mosapride also exerts anti-inflammatory effects on the gastrointestinal tract which may contribute to some of its therapeutic effects. Mosapride also promotes neurogenesis in the gastrointestinal tract which may prove useful in certain bowel disorders. The neurogenesis is due to mosapride's effect on the 5-HT4 receptor where it acts as an agonist.Its common side effects include dry mouth, abdominal pain, dizziness, headache, insomnia, malaise, nausea, diarrhoea and sometimes constipation. Unlike some other prokinetic agents, mosapride has little effect on potassium channels, no effect on hERG transfected cells, and no effect on cardiovascular function that could be detected in tests on humans. Due to the pharmacokinetics of mosapride, it would take 1,000 - 3,000 times the therapeutic dose to elicit cardiovascular effects. |
| Name | Mosapride citrate |
|---|---|
| Synonym | More Synonyms |
| Description | Mosapride citrate is a gastroprokinetic agent that acts as a selective 5HT4 agonist.Target: 5HT4 Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastro-oesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).In addition to its prokinetic properties, mosapride also exerts anti-inflammatory effects on the gastrointestinal tract which may contribute to some of its therapeutic effects. Mosapride also promotes neurogenesis in the gastrointestinal tract which may prove useful in certain bowel disorders. The neurogenesis is due to mosapride's effect on the 5-HT4 receptor where it acts as an agonist.Its common side effects include dry mouth, abdominal pain, dizziness, headache, insomnia, malaise, nausea, diarrhoea and sometimes constipation. Unlike some other prokinetic agents, mosapride has little effect on potassium channels, no effect on hERG transfected cells, and no effect on cardiovascular function that could be detected in tests on humans. Due to the pharmacokinetics of mosapride, it would take 1,000 - 3,000 times the therapeutic dose to elicit cardiovascular effects. |
|---|---|
| Related Catalog | |
| References |
[2]. Curran MP, et al. Mosapride in gastrointestinal disorders. Drugs. 2008;68(7):981-91. |
| Boiling Point | 549.2ºC at 760mmHg |
|---|---|
| Molecular Formula | C27H33ClFN3O10 |
| Molecular Weight | 614.016 |
| Flash Point | 286ºC |
| Exact Mass | 613.183838 |
| PSA | 212.44000 |
| LogP | 2.93620 |
| Vapour Pressure | 4.11E-12mmHg at 25°C |
| Storage condition | Desiccate at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|
|
~86% 
Mosapride citrate CAS#:112885-42-4 |
| Literature: Richter Gedeon Vegyeszeti Gyar RT. Patent: WO2003/106440 A2, 2003 ; Location in patent: Page 10 ; |
|
~83% 
Mosapride citrate CAS#:112885-42-4 |
| Literature: Richter Gedeon Vegyeszeti Gyar RT. Patent: WO2003/106440 A2, 2003 ; Location in patent: Page 10 ; |
|
~96% 
Mosapride citrate CAS#:112885-42-4 |
| Literature: RANBAXY LABORATORIES LIMITED; HASHMI, Mohd Salman; MADHRA, Mukesh Kumar; IF2 Patent: WO2011/107903 A1, 2011 ; Location in patent: Page/Page column 11 ; |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| 4-Amino-5-chloro-2-ethoxy-N-{[4-(4-fluorobenzyl)morpholin-2-yl]methyl}benzamide 2-hydroxypropane-1,2,3-tricarboxylate (1:1) |
| (±)-4-Amino-5-chloro-2-ethoxy-N-((4-(4-fluorobenzyl)-2-morpholinyl)methyl)benzamide Citrate |
| 4-amino-5-chloro-2-ethoxy-N-{[4-(4-fluorobenzyl)morpholin-2-yl]methyl}benzamide 2-hydroxypropane-1,2,3-tricarboxylate (salt) |
| Benzamide (4-amino-5-chloro-2-ethoxy-N-[[4-[(4-fluorophenyl)methyl]-2-morpholinyl]methyl] |
| 4-Amino-5-chloro-2-ethoxy-N-{[4-(4-fluorobenzyl)-2-morpholinyl]methyl}benzamide 2-hydroxy-1,2,3-propanetricarboxylate (1:1) |
| Benzamide, 4-amino-5-chloro-2-ethoxy-N-[[4-[(4-fluorophenyl)methyl]-2-morpholinyl]methyl]-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1) (salt) |
| UNII:MF497J489P |
| 4-amino-5-chloro-2-ethoxy-N-[[4-[(4-fluorophenyl)methyl]morpholin-2-yl]methyl]benzamide,2-hydroxypropane-1,2,3-tricarboxylic acid |
| MFCD00874415 |
| Mosapride Citrate |
| Mosapride (citrate) |
| Mosapride Impurity 2 |