N-Cbz-L-Serine
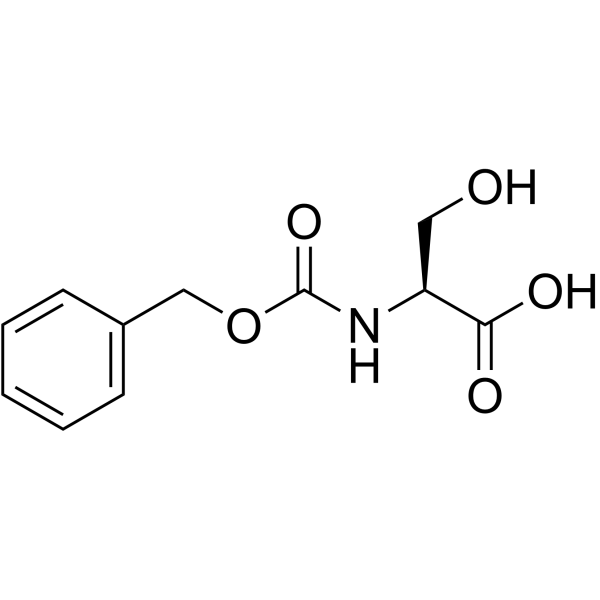
N-Cbz-L-Serine structure
|
Common Name | N-Cbz-L-Serine | ||
|---|---|---|---|---|
| CAS Number | 1145-80-8 | Molecular Weight | 239.225 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 487.5±45.0 °C at 760 mmHg | |
| Molecular Formula | C11H13NO5 | Melting Point | 116-119 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 248.6±28.7 °C | |
Use of N-Cbz-L-SerineZ-Ser-OH is a serine derivative[1]. |
| Name | N-Cbz-L-Serine |
|---|---|
| Synonym | More Synonyms |
| Description | Z-Ser-OH is a serine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 487.5±45.0 °C at 760 mmHg |
| Melting Point | 116-119 °C(lit.) |
| Molecular Formula | C11H13NO5 |
| Molecular Weight | 239.225 |
| Flash Point | 248.6±28.7 °C |
| Exact Mass | 239.079376 |
| PSA | 95.86000 |
| LogP | 0.83 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.572 |
| InChIKey | GNIDSOFZAKMQAO-VIFPVBQESA-N |
| SMILES | O=C(NC(CO)C(=O)O)OCc1ccccc1 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor.
Nat. Chem. Biol. 5 , 45-52, (2009) Transporter-related nutrient sensors, called transceptors, mediate nutrient activation of signaling pathways through the plasma membrane. The mechanism of action of transporting and nontransporting tr... |
|
|
Biochemical and mass spectrometric characterization of human N-acylethanolamine-hydrolyzing acid amidase inhibition.
PLoS ONE 7 , e43877, (2012) The mechanism of inactivation of human enzyme N-acylethanolamine-hydrolyzing acid amidase (hNAAA), with selected inhibitors identified in a novel fluorescent based assay developed for characterization... |
|
|
Analyte separation by OMNiMIPs imprinted with multiple templates.
Biosens. Bioelectron. 25(3) , 604-8, (2009) Changes detected in the imprinting effect by OMNiMIPs imprinted with multiple templates appear to be a function of the maximum template loading. Below the maximum template loading, the polymers imprin... |
| N-Carbobenzoxy-DL-serine |
| DL-Serine, N-[(phenylmethoxy)carbonyl]- |
| N-Carbobenzoxy-L-serine |
| Benzyloxycarbonyl-L-serine |
| L-Serine, N-[(phenylmethoxy)carbonyl]- |
| (2S)-3-hydroxy-2-(phenylmethoxycarbonylamino)propanoic acid |
| N-Benzyloxycarbonyl-L-serine |
| Serine, N-[(phenylmethoxy)carbonyl]- |
| EINECS 214-546-4 |
| L-Serine, N-benzyloxycarbonyl- |
| N-[(Benzyloxy)carbonyl]serine |
| Carbobenzyloxy-L-serine,Z-L-Serine |
| N-Cbz-L-serine |
| N-Carbobenzyloxy-L-serine |
| MFCD00002662 |
| Z-Ser-OH |
| CBZ-SER-OH |
 CAS#:56-45-1
CAS#:56-45-1 CAS#:501-53-1
CAS#:501-53-1 CAS#:21209-51-8
CAS#:21209-51-8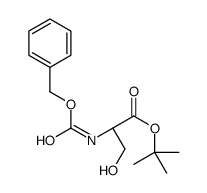 CAS#:59859-77-7
CAS#:59859-77-7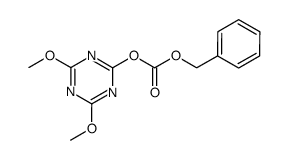 CAS#:419533-86-1
CAS#:419533-86-1 CAS#:60-29-7
CAS#:60-29-7 CAS#:312-84-5
CAS#:312-84-5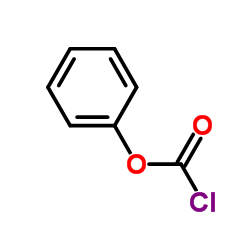 CAS#:1885-14-9
CAS#:1885-14-9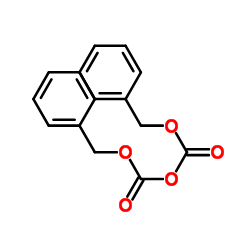 CAS#:31139-36-3
CAS#:31139-36-3 CAS#:33019-34-0
CAS#:33019-34-0 CAS#:19647-68-8
CAS#:19647-68-8 CAS#:19525-95-2
CAS#:19525-95-2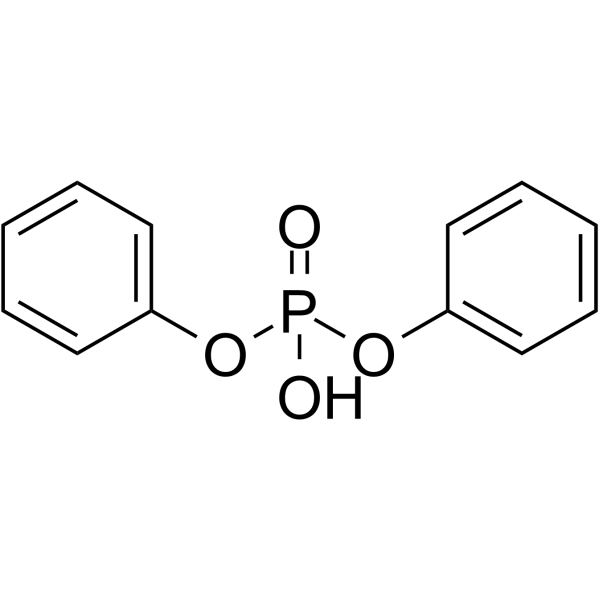 CAS#:838-85-7
CAS#:838-85-7 CAS#:16938-08-2
CAS#:16938-08-2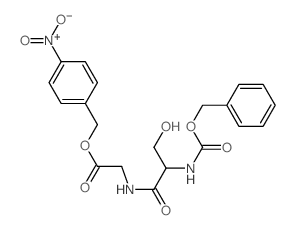 CAS#:26054-98-8
CAS#:26054-98-8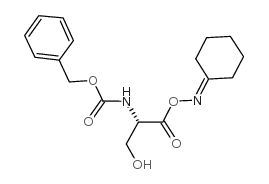 CAS#:24180-03-8
CAS#:24180-03-8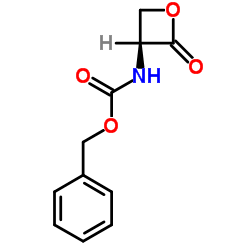 CAS#:26054-60-4
CAS#:26054-60-4
