N-Cbz-L-serine methyl ester
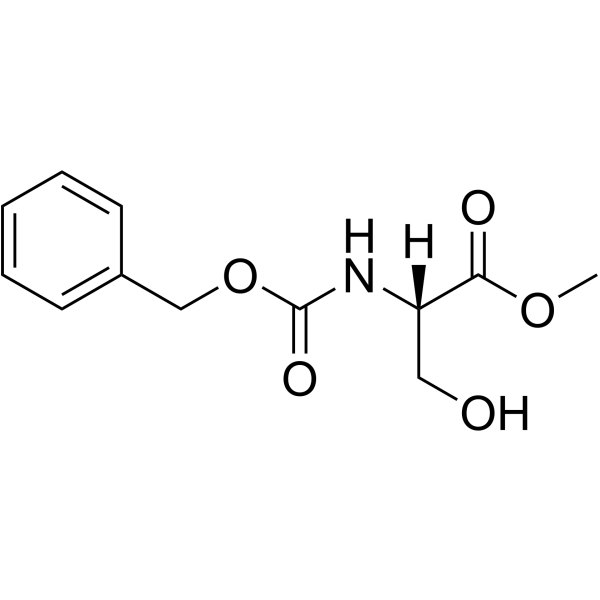
N-Cbz-L-serine methyl ester structure
|
Common Name | N-Cbz-L-serine methyl ester | ||
|---|---|---|---|---|
| CAS Number | 1676-81-9 | Molecular Weight | 253.251 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 442.6±45.0 °C at 760 mmHg | |
| Molecular Formula | C12H15NO5 | Melting Point | 41-43 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 221.5±28.7 °C | |
Use of N-Cbz-L-serine methyl esterZ-Ser-OMe is a serine derivative[1]. |
| Name | N-Z-L-serine methyl ester |
|---|---|
| Synonym | More Synonyms |
| Description | Z-Ser-OMe is a serine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 442.6±45.0 °C at 760 mmHg |
| Melting Point | 41-43 °C(lit.) |
| Molecular Formula | C12H15NO5 |
| Molecular Weight | 253.251 |
| Flash Point | 221.5±28.7 °C |
| Exact Mass | 253.095016 |
| PSA | 84.86000 |
| LogP | 1.42 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.536 |
| InChIKey | CINAUOAOVQPWIB-JTQLQIEISA-N |
| SMILES | COC(=O)C(CO)NC(=O)OCc1ccccc1 |
| Storage condition | -15°C |
| RIDADR | NONH for all modes of transport |
|---|---|
| WGK Germany | 3 |
| HS Code | 2924299090 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Stereoselective Synthesis of Differentially Protected Derivatives of the Higher Amino Sugars Destomic Acid and Lincosamine from Serine and Threonine.
J. Org. Chem. 61 , 581, (1996) The aminoheptose destomic acid (3.5) and the aminooctose lincosamine (6.8) were synthesized in protected form by parallel sequences starting from the oxazolidine derivatives 2.4 and 5.1 of N-CBz serin... |
|
|
Monache, G.D. et al.
Synthesis , 1155, (1995)
|
|
|
Demirci, F. et al.
Synthesis , 189, (1996)
|
| L-Serine, N-[(phenylmethoxy)carbonyl]-, methyl ester |
| Methyl N-[(benzyloxy)carbonyl]-L-serinate |
| N-Carbobenzyloxy-L-serine methyl ester |
| N-Carbobenzoxy-L-serine Methyl Ester |
| CBZ-L-Serine methyl ester |
| N-Cbz-L-serine Methyl Ester |
| N-Benzyloxycarbonyl-l-serine, methyl ester |
| MFCD00077039 |
| Cbz-Ser-OMe |
| (S)-Methyl 2-(((benzyloxy)carbonyl)amino)-3-hydroxypropanoate |
| methyl (2S)-3-hydroxy-2-(phenylmethoxycarbonylamino)propanoate |
| Z-Ser-OMe |
 CAS#:5680-80-8
CAS#:5680-80-8 CAS#:501-53-1
CAS#:501-53-1 CAS#:1145-80-8
CAS#:1145-80-8 CAS#:74-88-4
CAS#:74-88-4 CAS#:67-56-1
CAS#:67-56-1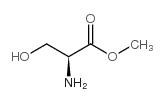 CAS#:2788-84-3
CAS#:2788-84-3 CAS#:186581-53-3
CAS#:186581-53-3 CAS#:77-78-1
CAS#:77-78-1![benzyl triazolo[4,5-b]pyridine-1-carboxylate Structure](https://image.chemsrc.com/caspic/139/108655-52-3.png) CAS#:108655-52-3
CAS#:108655-52-3 CAS#:35761-26-3
CAS#:35761-26-3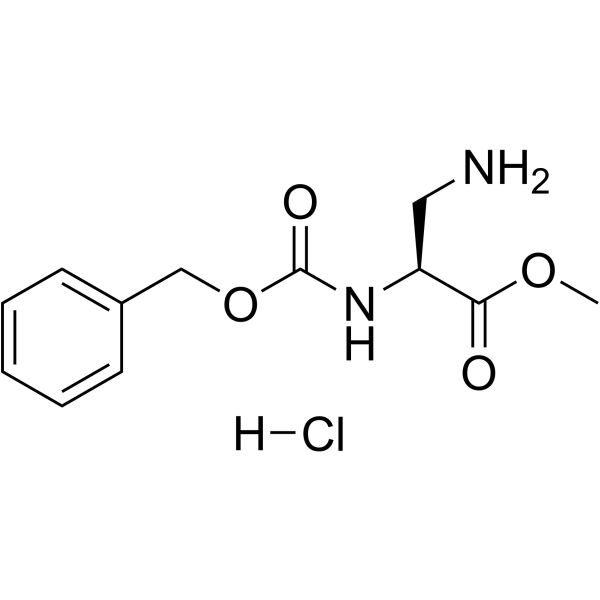 CAS#:35761-27-4
CAS#:35761-27-4 CAS#:19647-68-8
CAS#:19647-68-8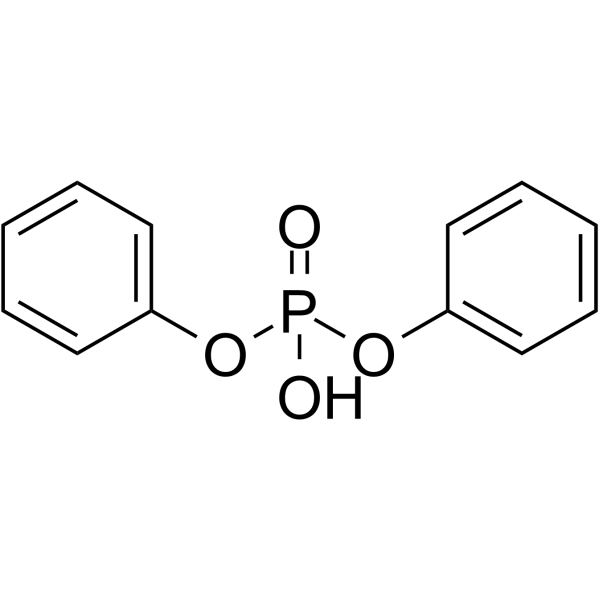 CAS#:838-85-7
CAS#:838-85-7![L-Serine,N-[(phenylmethoxy)carbonyl]-O-[3,4,6-tri-O-acetyl-2-(acetylamino)-2-deoxy-b-D-glucopyranosyl]-, methyl ester structure](https://image.chemsrc.com/caspic/393/25644-83-1.png) CAS#:25644-83-1
CAS#:25644-83-1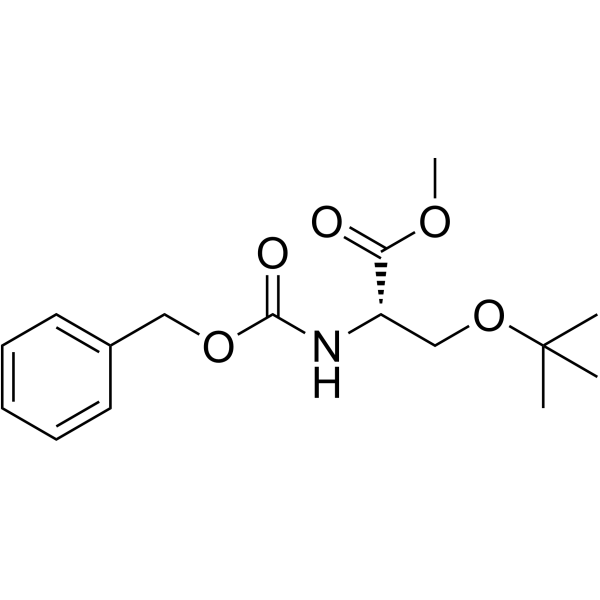 CAS#:1872-59-9
CAS#:1872-59-9 CAS#:17331-87-2
CAS#:17331-87-2 CAS#:30735-20-7
CAS#:30735-20-7
