| Description |
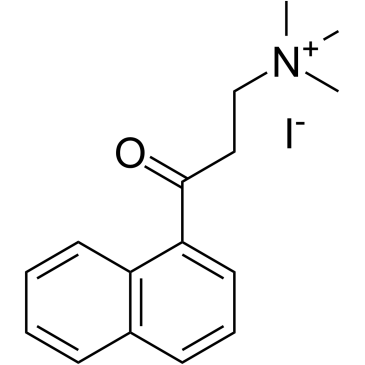
α-NETA is a potent and noncompetitive choline acetyltransferase (ChA) inhibitor with an IC50 of 9 μM. α-NETA is a potent ALDH1A1 (IC50=0.04 µM) and chemokine-like receptor-1 (CMKLR1) antagonist. α-NETA weakly inhibits cholinesterase (ChE; IC50=84 µM) and acetylcholinesterase (AChE; IC50=300 µM). α-NETA has anti-cancer activity[1][2].
|
| Related Catalog |
|
| Target |
IC50: 9 μM (ChAT); 0.04 µM (ALDH1A1); CMKLR1; 84 µM (ChE); 300 µM (AChE)[1][2]
|
| In Vitro |
α-NETA (50-150 nM; 24 hours) decreases all cell lines viability in a dose-dependent manner[3]. α-NETA (2.5-10.0 µg/mL; 24 hours) leads to epithelial ovarian cancer (EOC) cell death associated with membrane blistering and cytoplasm leakage[3]. α-NETA treatment increases EOC cell expression of pyroptosis-associated proteins[3]. α-NETA is most potent in inhibiting aldehyde dehydrogenase 1 family, member A1 (ALDH1A1; IC50=0.04 µM; purified enzymes assay), followed by CMKLR1 (IC50=0.375 µM for β-ARR2 recruitment; Cell-based assay) and G9a histone lysine methyltransferase (IC50=0.50 µM; purified enzymes assay). α-NETA selectively inhibits chemerin-stimulated CMKLR1 association with β-arrestin2[2]. α-NETA possesses fluorescent characteristics (excitation spectrum: maxima 255 and 297 nm; emission spectrum: maximum 437 nm) of naphthyl moiety[1]. Cell Viability Assay[3] Cell Line: Ho8910, Ho8910PM, A2780, and Iose80 cells Concentration: 50, 100, 150 nM Incubation Time: 24 hours Result: Decreased all cell lines viability in a dose-dependent manner. Apoptosis Analysis[3] Cell Line: Epithelial ovarian cancer (EOC) cell Concentration: 2.5, 7.5, 10.0 µg/mL Incubation Time: 24 hours Result: Led to EOC cell death associated with membrane blistering and cytoplasm leakage.
|
| In Vivo |
α-NETA (i.p.; 0.125 mg/kg; once every other day for 20 days) significantly decreases tumor volume and tumor weight[3]. α-NETA (s.c. injection; 3 mg/kg or 10 mg/kg; daily; for 30 days) significantly delays the onset of EAE with 3 mg/kg, and completely suppresses clinical signs for an average of nine days with 10 mg/kg beyond the first appearance of disease in control female C57BL/6 mice[2]. Animal Model: BALB/c nude mice with skov3 cells[3] Dosage: 0.125 mg/kg Administration: IP; once every other day for 20 days Result: Significantly decreased tumor volume and tumor weight.
|
| References |
[1]. Sastry BV, et al. Relationships between chemical structure and inhibition of choline acetyltransferase by 2-(alpha-naphthoyl)ethyltrimethylammonium and related compounds. Pharmacol Res Commun. 1988 Sep;20(9):751-71. [2]. Graham KL, et al. A novel CMKLR1 small molecule antagonist suppresses CNS autoimmune inflammatory disease. PLoS One. 2014 Dec 1;9(12):e112925. [3]. Qiao L, et al. α-NETA induces pyroptosis of epithelial ovarian cancer cells through the GSDMD/caspase-4 pathway. FASEB J. 2019 Nov;33(11):12760-12767.
|