Cinchonine
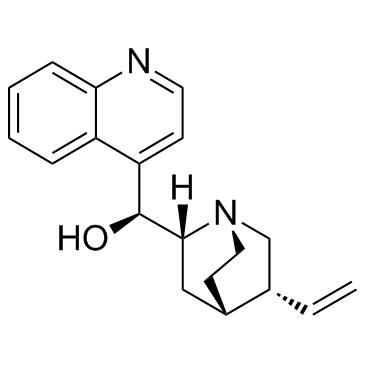
Cinchonine structure
|
Common Name | Cinchonine | ||
|---|---|---|---|---|
| CAS Number | 118-10-5 | Molecular Weight | 294.39 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 464.5±30.0 °C at 760 mmHg | |
| Molecular Formula | C19H22N2O | Melting Point | 260-263 °C | |
| MSDS | Chinese USA | Flash Point | 234.7±24.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CinchonineCinchonine is a natural compound present in Cinchona bark. Cinchonine activates endoplasmic reticulum stress-induced apoptosis in human liver cancer cells[1]. |
| Name | cinchonine |
|---|---|
| Synonym | More Synonyms |
| Description | Cinchonine is a natural compound present in Cinchona bark. Cinchonine activates endoplasmic reticulum stress-induced apoptosis in human liver cancer cells[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 464.5±30.0 °C at 760 mmHg |
| Melting Point | 260-263 °C |
| Molecular Formula | C19H22N2O |
| Molecular Weight | 294.39 |
| Flash Point | 234.7±24.6 °C |
| Exact Mass | 294.173218 |
| PSA | 36.36000 |
| LogP | 3.35 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.652 |
| InChIKey | KMPWYEUPVWOPIM-KAGGUIQQSA-N |
| SMILES | C=CC1CN2CCC1CC2C(O)c1ccnc2ccccc12 |
| Water Solubility | Insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H332 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S26-S36-S36/37 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | GD3500000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
~53% 
Cinchonine CAS#:118-10-5 |
| Literature: Journal of Organic Chemistry, , vol. 69, # 9 p. 2983 - 2991 |
|
~14% 
Cinchonine CAS#:118-10-5 
Cinchonine CAS#:118-10-5 |
| Literature: Arkivoc, , vol. 2012, # 6 p. 264 - 280 |
|
~30% 
Cinchonine CAS#:118-10-5 |
| Literature: Arkivoc, , vol. 2012, # 6 p. 264 - 280 |
|
~% 
Cinchonine CAS#:118-10-5 |
| Literature: Bulletin des Societes Chimiques Belges, , vol. 106, # 2 p. 77 - 84 |

Cinchonine CAS#:118-10-5 ~% 
Cinchonine CAS#:118-10-5 |
| Literature: Bulletin des Societes Chimiques Belges, , vol. 106, # 2 p. 77 - 84 |

Cinchonine CAS#:118-10-5 ~% 
Cinchonine CAS#:118-10-5 |
| Literature: Arkivoc, , vol. 2012, # 6 p. 264 - 280 |

Cinchonine CAS#:118-10-5 ~% 
Cinchonine CAS#:118-10-5
Detail
|
| Literature: Justus Liebigs Annalen der Chemie, , vol. 492, p. 242,255 |
| Precursor 4 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Multi-target spectral moment QSAR versus ANN for antiparasitic drugs against different parasite species.
Bioorg. Med. Chem. 18 , 2225-31, (2010) There are many of pathogen parasite species with different susceptibility profile to antiparasitic drugs. Unfortunately, almost QSAR models predict the biological activity of drugs against only one pa... |
|
|
Mutation in the Plasmodium falciparum CRT protein determines the stereospecific activity of antimalarial cinchona alkaloids.
Antimicrob. Agents Chemother. 56(10) , 5356-64, (2012) The Cinchona alkaloids are quinoline aminoalcohols that occur as diastereomer pairs, typified by (-)-quinine and (+)-quinidine. The potency of (+)-isomers is greater than the (-)-isomers in vitro and ... |
|
|
Thousands of chemical starting points for antimalarial lead identification.
Nature 465 , 305-10, (2010) Malaria is a devastating infection caused by protozoa of the genus Plasmodium. Drug resistance is widespread, no new chemical class of antimalarials has been introduced into clinical practice since 19... |
| (1S)-Quinolin-4-yl((2R,4S,5R)-5-vinylquinuclidin-2-yl)methanol |
| homoquinidine |
| MFCD00064372 |
| EINECS 204-234-6 |
| cinchonidine |
| chinchonine |
| Cinchonine |
| Cinchonan-9-ol, (8α,9S)- |
| D-CINCHONINE |
| Cinchonin |
| (8α,9S)-Cinchonan-9-ol |
![methanesulfonic acid (quinolin-4-yl)(5-vinyl-1-azabicyclo[2.2.2]oct-2-yl)methyl ester structure](https://image.chemsrc.com/caspic/054/560119-43-9.png)
![(1S,2R,3S,5S,6R)-2-(quinolin-4-yl)-6-vinyl-1-azabicyclo[3.2.2]nonan-3-ol structure](https://image.chemsrc.com/caspic/468/560119-45-1.png)


 CAS#:32644-15-8
CAS#:32644-15-8 CAS#:10009-70-8
CAS#:10009-70-8 CAS#:536-78-7
CAS#:536-78-7![(1S)-quinolin-4-yl((2R,4S,5R)-5-vinylquinuclidin-2-yl)methanol (S)-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)acetate structure](https://image.chemsrc.com/caspic/424/1007128-30-4.png) CAS#:1007128-30-4
CAS#:1007128-30-4 CAS#:324757-50-8
CAS#:324757-50-8 CAS#:490-11-9
CAS#:490-11-9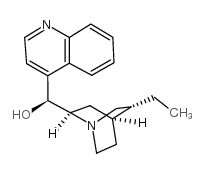 CAS#:485-65-4
CAS#:485-65-4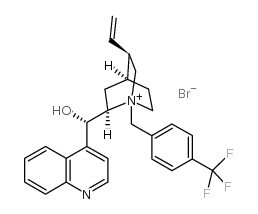 CAS#:95088-20-3
CAS#:95088-20-3
