Cinchonine
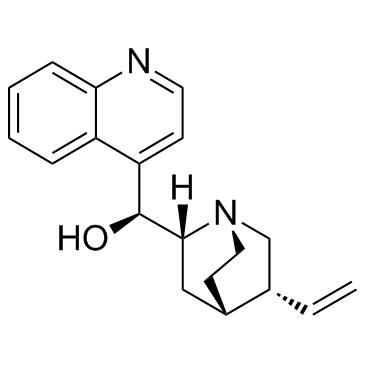
Cinchonine structure
|
Common Name | Cinchonine | ||
|---|---|---|---|---|
| CAS Number | 118-10-5 | Molecular Weight | 294.39 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 464.5±30.0 °C at 760 mmHg | |
| Molecular Formula | C19H22N2O | Melting Point | 260-263 °C | |
| MSDS | Chinese USA | Flash Point | 234.7±24.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Multi-target spectral moment QSAR versus ANN for antiparasitic drugs against different parasite species.
Bioorg. Med. Chem. 18 , 2225-31, (2010) There are many of pathogen parasite species with different susceptibility profile to antiparasitic drugs. Unfortunately, almost QSAR models predict the biological activity of drugs against only one parasite species. Consequently, predicting the probability wi... |
|
|
Mutation in the Plasmodium falciparum CRT protein determines the stereospecific activity of antimalarial cinchona alkaloids.
Antimicrob. Agents Chemother. 56(10) , 5356-64, (2012) The Cinchona alkaloids are quinoline aminoalcohols that occur as diastereomer pairs, typified by (-)-quinine and (+)-quinidine. The potency of (+)-isomers is greater than the (-)-isomers in vitro and in vivo against Plasmodium falciparum malaria parasites. Th... |
|
|
Thousands of chemical starting points for antimalarial lead identification.
Nature 465 , 305-10, (2010) Malaria is a devastating infection caused by protozoa of the genus Plasmodium. Drug resistance is widespread, no new chemical class of antimalarials has been introduced into clinical practice since 1996 and there is a recent rise of parasite strains with redu... |
|
|
Discovery of potent small-molecule inhibitors of multidrug-resistant Plasmodium falciparum using a novel miniaturized high-throughput luciferase-based assay.
Antimicrob. Agents Chemother. 54 , 3597-604, (2010) Malaria is a global health problem that causes significant mortality and morbidity, with more than 1 million deaths per year caused by Plasmodium falciparum. Most antimalarial drugs face decreased efficacy due to the emergence of resistant parasites, which ne... |
|
|
A theoretical investigation on the Strecker reaction catalyzed by a Ti(IV)-complex catalyst generated from a cinchona alkaloid, achiral substituted 2,2'-biphenol, and tetraisopropyl titanate.
Chemistry 19(5) , 1637-46, (2013) The mechanism and the origin of selectivity of the asymmetric Strecker reaction catalyzed by a Ti(IV)-complex catalyst generated from a cinchona alkaloid, achiral substituted 2,2'-biphenol, and tetraisopropyl titanate have been investigated by DFT and ONIOM m... |
|
|
Organocatalytic asymmetric conjugate addition of 3-monosubstituted oxindoles to (E)-1,4-diaryl-2-buten-1,4-diones: a strategy for the indirect enantioselective furanylation and pyrrolylation of 3-alkyloxindoles.
Chemistry 18(21) , 6679-87, (2012) An asymmetric conjugate addition of 3-monosubstituted oxindoles to a range of (E)-1,4-diaryl-2-buten-1,4-diones, catalyzed by commercially available cinchonine, is described. This organocatalytic asymmetric reaction affords a broad range of 3,3'-disubstituted... |
|
|
Synthesis and optical resolution of an allenoic acid by diastereomeric salt formation induced by chiral alkaloids.
Chirality 20(1) , 47-50, (2008) A synthetic procedure for the preparation of 4-cyclohexyl-2-methyl-buta-2,3-dienoic acid in the two optically active forms has been developed. Synthesis of the racemic allenoic acid was made by an efficient route with good overall yield. Resolution of the ena... |
|
|
Self-association promoted conformational transition of (3R,4S,8R,9R)-9-[(3,5-bis(trifluoromethyl)phenyl))-thiourea](9-deoxy)-epi-cinchonine.
Magn. Reson. Chem. 48(1) , 13-9, (2010) The conformational diversity of the (3R,4S,8R,9R)-9-[(3,5-bis(trifluoromethyl)phenyl))-thiourea](9-deoxy)-epi-cinchonine organocatalyst is discussed. Low-temperature NMR experiments confirmed a self-association process, which promotes the quinoline rotation b... |
|
|
An improved synthesis of 10,11-didehydro Cinchona alkaloids.
Chirality 20(3-4) , 441-5, (2008) A revised procedure for the conversion of the four major Cinchona alkaloids (quinine, quinidine, cinchonidine, and cinchonine) into their respective 10,11-didehydro derivatives is described. The reported protocol offers several advantages over a recently publ... |
|
|
Hydrocinchonine, cinchonine, and quinidine potentiate paclitaxel-induced cytotoxicity and apoptosis via multidrug resistance reversal in MES-SA/DX5 uterine sarcoma cells.
Environ. Toxicol. 26(4) , 424-31, (2011) Multidrug resistance (MDR) is one of important issues to cause the chemotherapy failure against cancers including gynecological malignancies. Despite some MDR reversal evidences of natural compounds including quinidine and cinchonine, there are no reports on ... |