EXP 3174
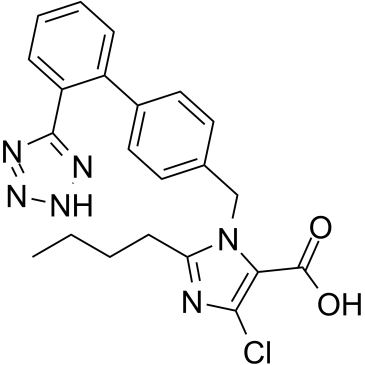
EXP 3174 structure
|
Common Name | EXP 3174 | ||
|---|---|---|---|---|
| CAS Number | 124750-92-1 | Molecular Weight | 436.894 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 707.8±70.0 °C at 760 mmHg | |
| Molecular Formula | C22H21ClN6O2 | Melting Point | 130-132ºC | |
| MSDS | N/A | Flash Point | 381.8±35.7 °C | |
Use of EXP 3174Losartan Carboxylic Acid (E-3174), an active carboxylic acid metabolite of Losartan, is an angiotensin II receptor type 1 (AT1) antagonist. The Ki values are 0.97, 0.57, 0.67 nM for rat AT1B/AT1A and human AT1, respectively. Losartan Carboxylic Acid blocks the angiotensin II-induced responses in vascular smoothmuscle cells (VSMC). Losartan Carboxylic Acid elevates plasma renin activities and reduces mean arterial pressure[1][2][3][4]. |
| Name | 2-butyl-5-chloro-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Losartan Carboxylic Acid (E-3174), an active carboxylic acid metabolite of Losartan, is an angiotensin II receptor type 1 (AT1) antagonist. The Ki values are 0.97, 0.57, 0.67 nM for rat AT1B/AT1A and human AT1, respectively. Losartan Carboxylic Acid blocks the angiotensin II-induced responses in vascular smoothmuscle cells (VSMC). Losartan Carboxylic Acid elevates plasma renin activities and reduces mean arterial pressure[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Angiotensin II receptor type 1[1] |
| In Vitro | The specific binding of [125I]-angiotensin II to VSMC is inhibited by Losartan Carboxylic Acid (E-3174) with an IC50 of 1.1 nM. Losartan Carboxylic Acid abolishes the angiotensin II-induced formation of inositolphosphates in VSMC. Losartan Carboxylic Acid inhibits the angiotensin II-induced elevation of intracellular cytosolic Ca2+ concentration with an IC50 of 5 nM. Losartan Carboxylic Acid is more effective than losartan in blocking the angiotensin II-induced increase in Egr-1 mRNA. Losartan Carboxylic Acid inhibits the angiotensin II-induced cell protein synthesis with an IC50 of 3 nM[1]. |
| In Vivo | Losartan Carboxylic Acid (E-3174) (0.1 mg/kg; i.v. followed by 0.02 mg/kg/h for 5.5 h) induces a similar level of inhibition (87±4%) of the pressor responses to angiotensin I[3]. Intravenous Losartan Carboxylic Acid (0.1 mg/kg+0.01 mg/kg/min) is infused in anesthetized dogs with recent (8.1±0.4 days) anterior myocardial infarction. Electrolytic injury of the left circumflex coronary artery to induce thrombotic occlusion and posterolateral ischemia is initiated 1 h after the start of treatment[4]. Animal Model: Mongrel dogs of either sex, weighing 15-25 kg[3] Dosage: 0.1 mg/kg (followed by 0.02 mg/kg/h) Administration: i.v. for 5.5 hours Result: The pressor response was reduced by 87±4%. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 707.8±70.0 °C at 760 mmHg |
| Melting Point | 130-132ºC |
| Molecular Formula | C22H21ClN6O2 |
| Molecular Weight | 436.894 |
| Flash Point | 381.8±35.7 °C |
| Exact Mass | 436.141449 |
| PSA | 109.58000 |
| LogP | 4.79 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.695 |
| InChIKey | ZEUXAIYYDDCIRX-UHFFFAOYSA-N |
| SMILES | CCCCc1nc(Cl)c(C(=O)O)n1Cc1ccc(-c2ccccc2-c2nn[nH]n2)cc1 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | 24/25 |
|---|
|
~89% 
EXP 3174 CAS#:124750-92-1 |
| Literature: SHANGHAI ALLIST PHARMACEUTICAL., INC. Patent: EP1988090 A1, 2008 ; Location in patent: Page/Page column 11 ; EP 1988090 A1 |
|
~78% 
EXP 3174 CAS#:124750-92-1 |
| Literature: Chen, Liqin; Li, Jian; Shen, Yingzhong Patent: US2008/90885 A1, 2008 ; Location in patent: Page/Page column 2 ; |
|
~64% 
EXP 3174 CAS#:124750-92-1 |
| Literature: Tetrahedron Letters, , vol. 44, # 6 p. 1149 - 1152 |
| 2-n-butyl-5-chloro-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-imidazole-4-carboxylic acid |
| E-3174 |
| Losartan carboxylic acid |
| 1H-Imidazole-5-carboxylic acid, 2-butyl-4-chloro-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]- |
| 2-Butyl-4-chloro-1-{[2'-(1H-tetrazol-5-yl)-4-biphenylyl]methyl}-1H-imidazole-5-carboxylic acid |
| Carboxylosartan |
| 2-Butyl-4-chloro-1-{[2'-(2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazole-5-carboxylic acid |
| 1H-Imidazole-5-carboxylic acid,2-butyl-4-chloro-1-((2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl) |
| Exp3174 |
| EXP-3174 |

![2-butyl-4-chloro-1-[(2'-(1H-tetrazol-5-yl) (1,1'-biphenyl)-4-yl)methyl]-1H-imidazole-5-carboxylic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester structure](https://image.chemsrc.com/caspic/482/1070174-98-9.png) CAS#:1070174-98-9
CAS#:1070174-98-9 CAS#:2387-23-7
CAS#:2387-23-7![2-butyl-4-chloro-1-[(2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl]-1H-imidazole-5-carboxylic acid 2-(methylsulfonylthio)ethyl ester structure](https://image.chemsrc.com/caspic/434/1070174-99-0.png) CAS#:1070174-99-0
CAS#:1070174-99-0![2-butyl-5-chloro-3-[[4-[2-(1-trityltetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylic acid structure](https://image.chemsrc.com/caspic/134/947331-10-4.png) CAS#:947331-10-4
CAS#:947331-10-4