Ulipristal acetate
Modify Date: 2025-08-20 19:56:48
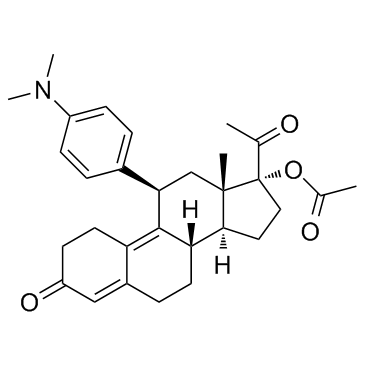
Ulipristal acetate structure
|
Common Name | Ulipristal acetate | ||
|---|---|---|---|---|
| CAS Number | 126784-99-4 | Molecular Weight | 475.619 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 640.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C30H37NO4 | Melting Point | 183-185ºC | |
| MSDS | USA | Flash Point | 340.9±31.5 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of Ulipristal acetateUlipristal (acetate) is a novel selective progesterone receptor modulator (SPRM) for the treatment of benign gynecological conditions such as uterine myoma. |
| Name | ulipristal acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Ulipristal (acetate) is a novel selective progesterone receptor modulator (SPRM) for the treatment of benign gynecological conditions such as uterine myoma. |
|---|---|
| Related Catalog | |
| In Vitro | Ulipristal acetate blocks activin A modulation of fibronectin and vascular endothelial growth factor A (VEGF-A) mRNA expression in cultured myometrial and leiomyoma cells[2]. Ulipristal acetate decreases the DNA fragmentation at the 100-ng/mL dose and continuing up to the 10,000-ng/mL dose compared to those spermatozoa in the control group[3]. |
| In Vivo | Ulipristal and CDB-4124 have significant antiprogestational activity in vivo[1]. Ulipristal acetate decreases incidences of fibroadenomas and adenocarcinomas in the mammary gland in all treated groups. Ulipristal acetate exposure [AUC(0-24h)] at the highest dose in rats is 67 times human therapeutic exposure at 10 mg/day. In mice, no tumor of any type increases at Ulipristal acetate exposures up to 313 times of therapeutic exposure. Ulipristal acetate-related findings in mice are limited to organ weight changes in the liver, pituitary, thyroid/parathyroid glands, and epididymis as well as minimal panlobular hepatocellular hypertrophy in male and female mice receiving 130 mg/kg/day[4]. Ulipristal acetate (1 mg/kg and 5 mg/kg) increases the frequency with which pathologists assessed the endometrium as being thickened compared to controls in a dose-dependent manner. There is a slight decrease in secretory differentiation with increasing dose of Ulipristal acetate, with small decreases in frequency of sub- and supra-nuclear vacuolation[5]. |
| Animal Admin | The study consisted of four groups, each comprising four female cynomolgus monkeys. The groups eitherreceive ASV (control), or Ulipristal acetate at dose levels of 1, 5, or 25 mg/kg for 39 weeks. Two additional animals are allocated to the control and high dose groups for an 8-week post-dose recovery period. At randomization, there is no statistically significant difference between treatment groups in mean body weight. The vehicle or Ulipristal acetate is administered to all groups by oral gavage for 273 consecutive days at a dose volume of 2 mL/kg. Following the dosing or recovery period, animals are euthanized by intravenous administration of sodium pentobarbital followed by exsanguination of the femoral vessels. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 640.1±55.0 °C at 760 mmHg |
| Melting Point | 183-185ºC |
| Molecular Formula | C30H37NO4 |
| Molecular Weight | 475.619 |
| Flash Point | 340.9±31.5 °C |
| Exact Mass | 475.272247 |
| PSA | 63.68000 |
| LogP | 4.48 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.594 |
| InChIKey | OOLLAFOLCSJHRE-ZHAKMVSLSA-N |
| SMILES | CC(=O)OC1(C(C)=O)CCC2C3CCC4=CC(=O)CCC4=C3C(c3ccc(N(C)C)cc3)CC21C |
| Storage condition | -20°C |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| [(8S,11R,13S,14S,17R)-17-acetyl-11-[4-(dimethylamino)phenyl]-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] acetate |
| (11β)-17-(Acetyloxy)-11-(4-(dimethylamino)phenyl)-19-norpregna-4,9-diene-3,20-dione |
| (11β)-11-[4-(Dimethylamino)phenyl]-3,20-dioxo-19-norpregna-4,9-dien-17-yl acetate |
| Estra-4,9-dien-3-one, 17-acetyl-17-(acetyloxy)-11-[4-(dimethylamino)phenyl]-, (11β,17α)- |
| Ulipristal acetate |
| VA2914 |
| 19-Norpregna-4,9-diene-3,20-dione, 17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-, (11β)- |
| Ella |
| CDB-2914 |
| Ulipristal (acetate) |
 CAS#:730962-28-4
CAS#:730962-28-4 CAS#:108-24-7
CAS#:108-24-7 CAS#:159811-51-5
CAS#:159811-51-5 CAS#:96-63-9
CAS#:96-63-9 CAS#:126690-41-3
CAS#:126690-41-3![(5α,11β,17α)-11-[4-(dimethylamino)phenyl]-5,17-dihydroxy-19-norpregn-9-en-20-yn-3-one Cyclic 1,2-Ethanediyl Acetal Structure](https://image.chemsrc.com/caspic/359/91934-95-1.png) CAS#:91934-95-1
CAS#:91934-95-1 CAS#:54201-84-2
CAS#:54201-84-2 CAS#:54201-83-1
CAS#:54201-83-1 CAS#:290825-36-4
CAS#:290825-36-4
