Radicicol
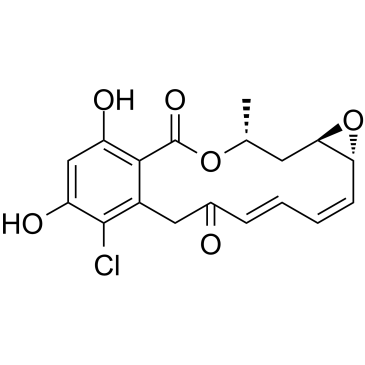
Radicicol structure
|
Common Name | Radicicol | ||
|---|---|---|---|---|
| CAS Number | 12772-57-5 | Molecular Weight | 364.777 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 656.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C18H17ClO6 | Melting Point | 190-194ºC | |
| MSDS | Chinese USA | Flash Point | 350.7±31.5 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of RadicicolRadicicol is an inhibitor of the ATPase/kinase with IC50 values of 1 μM, 100 μM and 400 μM, respectively for Hsp90, Topo VI and PDK3 [1]. Radicicol inhibits a wide variety of tumor cell lines by targeting Hsp90, binds to the ATPase domain of Hsp90 and prevents maturation of Hsp90 clients, leading to proteasomal degradation [2]. Radicicol is an antifungal antibiotic with antimalarial activity, impairs mitochondrial replication by targeting P. falciparum topoisomerase VIB[2]. Radicicol is an inhibitor of fat mass and obesity-associated protein (FTO), exhibits a dose-dependent inhibition of FTO demethylation activity with an IC50 value of 16.04 µM[3]. |
| Name | radicicol |
|---|---|
| Synonym | More Synonyms |
| Description | Radicicol is an inhibitor of the ATPase/kinase with IC50 values of 1 μM, 100 μM and 400 μM, respectively for Hsp90, Topo VI and PDK3 [1]. Radicicol inhibits a wide variety of tumor cell lines by targeting Hsp90, binds to the ATPase domain of Hsp90 and prevents maturation of Hsp90 clients, leading to proteasomal degradation [2]. Radicicol is an antifungal antibiotic with antimalarial activity, impairs mitochondrial replication by targeting P. falciparum topoisomerase VIB[2]. Radicicol is an inhibitor of fat mass and obesity-associated protein (FTO), exhibits a dose-dependent inhibition of FTO demethylation activity with an IC50 value of 16.04 µM[3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: of 1 μM (Hsp90), 100 μM (Topo VI) and 400 μM (PDK3)[1] |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 656.2±55.0 °C at 760 mmHg |
| Melting Point | 190-194ºC |
| Molecular Formula | C18H17ClO6 |
| Molecular Weight | 364.777 |
| Flash Point | 350.7±31.5 °C |
| Exact Mass | 364.071381 |
| PSA | 96.36000 |
| LogP | 1.53 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.583 |
| InChIKey | WYZWZEOGROVVHK-WSGSENCDSA-N |
| SMILES | CC1CC2OC2C=CC=CC(=O)Cc2c(Cl)c(O)cc(O)c2C(=O)O1 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H340-H350 |
| Precautionary Statements | Missing Phrase - N15.00950417-P201-P280-P308 + P313 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 1 |
| RTECS | RR1105000 |
| Hazard Class | 6.1 |
| HS Code | 29322090 |
|
Screening for negative effects of candidate ascidian antifoulant compounds on a target aquaculture species, Perna canaliculus Gmelin.
Biofouling 29(1) , 29-37, (2013) The natural chemical compounds radicicol, polygodial and ubiquinone-10 (Q10) have previously been identified as inhibitors of metamorphosis in ascidian larvae. Accordingly, they have potential as a sp... |
|
|
[Thermosensitization of tumor cells with inhibitors of chaperone activity and expression].
Biomed. Khim. 58(6) , 662-72, (2012) Effects of inhibitors of the heat shock protein 90 (HSP90) chaperone activity and inhibitors of the heat shock protein (HSP) expression on sensitivity of HeLa tumor cells to hyperthermia were studied.... |
|
|
Geldanamycin and its derivatives as Hsp90 inhibitors.
Front. Biosci. (Landmark Ed.) 17 , 2269-77, (2012) The Hsp90 molecule, one of the most abundant heat shock proteins in mammalian cells, maintains homeostasis and prevents stress-induced cellular damage. Hsp90 is expressed under normal conditions at a ... |
| monorden |
| monorderne |
| [Biotinyl]-Radicicol |
| (1aR,2Z,4E,14R,15aR)-8-Chloro-9,11-dihydroxy-14-methyl-1a,14,15,15a-tetrahydro-6H-oxireno[e][2]benzoxacyclotetradecine-6,12(7H)-dione |
| Monorden/Radicicol |
| humicola fuscoatra |
| Radicicol from Diheterospora chlamydosporia |
| 6H-Oxireno[e][2]benzoxacyclotetradecin-6,12(7H)-dione, 8-chloro-1a,14,15,15a-tetrahydro-9,11-dihydroxy-14-methyl-, (1aR,2Z,4E,14R,15aR)- |
| MFCD01709459 |
| KF9-A |
| RADISICOL |
| Radicicol |
| [TAMRA]-Radicicol |
| MONORODENE |
| FO-4910 |
 CAS#:75207-13-5
CAS#:75207-13-5 CAS#:378749-88-3
CAS#:378749-88-3 CAS#:378750-02-8
CAS#:378750-02-8 CAS#:480-64-8
CAS#:480-64-8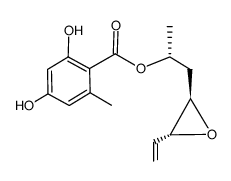 CAS#:867367-94-0
CAS#:867367-94-0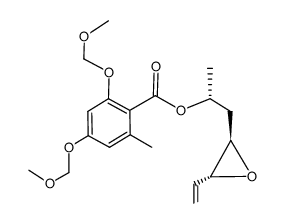 CAS#:867367-83-7
CAS#:867367-83-7![(1aR,2Z,4E,14R,15aR)-9,11-bis(methoxymethoxy)-14-methyl-1a,14,15,15a-tetrahydro-6H-benzo[c]oxireno[2,3-k][1]oxacyclotetradecine-6,12(7H)-dione Structure](https://image.chemsrc.com/caspic/254/754201-18-8.png) CAS#:754201-18-8
CAS#:754201-18-8![(1aR,2Z,4E,14R,15aR)-9,11-bis(methoxymethoxy)-14-methyl-1a,14,15,15a-tetrahydro-6H-benzo[c]oxireno[2,3-k][1]oxacyclotetradecine-6,12(7H)-dione Structure](https://image.chemsrc.com/caspic/275/754201-22-4.png) CAS#:754201-22-4
CAS#:754201-22-4
