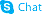4-Trifluoromethylphenylboronic acid
4-Trifluoromethylphenylboronic acid structure
|
Common Name | 4-Trifluoromethylphenylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 128796-39-4 | Molecular Weight | 189.93 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 258.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C7H6BF3O2 | Melting Point | 245-250 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 110.2±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-Trifluoromethylphenylboronic acid4-Trifluoromethylphenylboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-Trifluoromethylphenylboronic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Trifluoromethylphenylboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 4-Trifluoromethylphenylboronic acid 用于通过铜交换的氟磷灰石对咪唑和胺进行 N-芳基化,以及用于微波促进的与酰氯的交叉偶联,从而生成芳基酮。 |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 258.6±50.0 °C at 760 mmHg |
| Melting Point | 245-250 °C(lit.) |
| Molecular Formula | C7H6BF3O2 |
| Molecular Weight | 189.93 |
| Flash Point | 110.2±30.1 °C |
| Exact Mass | 190.041290 |
| PSA | 40.46000 |
| LogP | 2.16 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.462 |
| Storage condition | 0-6°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2931900090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combin... |
|
|
Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramolecular C-H amidation sequence: a novel route to 4-aryl-2-quinolinones.
Chem. Commun. (Camb.) 36th ed., 48 , 4332-4334, (2012) A novel catalytic method for synthesizing 4-aryl-2-quinolinones is reported. The process involves two mechanistically independent, sequential Pd(II)-catalyzed reactions--the oxidative Heck reaction an... |
|
|
Combined experimental-theoretical NMR study on 2,5-bis(5-aryl-3-hexylthiophen-2-yl)-thiazolo[5,4-d]thiazole derivatives for printable electronics.
Magn. Reson. Chem. 5th ed., 50 , 379-387, (2012) Four 2,5-bis(5-aryl-3-hexylthiophen-2-yl)thiazolo[5,4-d]thiazole derivatives have been synthesized and thoroughly characterized. The extended aromatic core of the molecules was designed to enhance the... |
| QBQR DXFFF |
| MFCD00151855 |
| 4-(Trifluoromethyl)phenylboronic acid |
| Boronic acid, B-[4-(trifluoromethyl)phenyl]- |
| α,α,α-Trifluoro-p-tolueneboronic Acid,contains varying amounts of An |
| α,α,α-Trifluoro-p-tolueneboronic Acid |
| Dihydroxy[4-(trifluoromethyl)phenyl]borane |
| α,α,α-Trifluoro-p-tolylboronic acid |
| (4-(Trifluoromethyl)phenyl)boronic acid |
| 4-(Trifluoromethyl)benzeneboronic acid |
| [4-(Trifluoromethyl)phenyl]boranediol |
| B-[4-(Trifluoromethyl)phenyl]boronic acid |
| [4-(Trifluoromethyl)phenyl]boronic acid |
| (4-Trifluoromethylphenyl)boronic acid |
 CAS#:402-43-7
CAS#:402-43-7 CAS#:121-43-7
CAS#:121-43-7 CAS#:5419-55-6
CAS#:5419-55-6 CAS#:7440-44-0
CAS#:7440-44-0 CAS#:1122-58-3
CAS#:1122-58-3 CAS#:23287-26-5
CAS#:23287-26-5![dimethoxy-[4-(trifluoromethyl)phenyl]borane Structure](https://www.chemsrc.com/caspic/201/873066-23-0.png) CAS#:873066-23-0
CAS#:873066-23-0 CAS#:98-56-6
CAS#:98-56-6 CAS#:98-08-8
CAS#:98-08-8 CAS#:108789-37-3
CAS#:108789-37-3 CAS#:455-14-1
CAS#:455-14-1![(4'-(TRIFLUOROMETHYL)-[1,1'-BIPHENYL]-4-YL)BORONIC ACID structure](https://www.chemsrc.com/caspic/205/364590-93-2.png) CAS#:364590-93-2
CAS#:364590-93-2 CAS#:395-23-3
CAS#:395-23-3 CAS#:583-02-8
CAS#:583-02-8 CAS#:455-24-3
CAS#:455-24-3 CAS#:457889-46-2
CAS#:457889-46-2 CAS#:142557-76-4
CAS#:142557-76-4 CAS#:447406-52-2
CAS#:447406-52-2 CAS#:90035-34-0
CAS#:90035-34-0