Eplivanserin hemifumarate
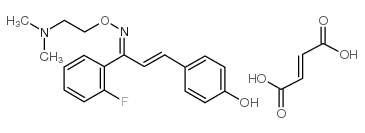
Eplivanserin hemifumarate structure
|
Common Name | Eplivanserin hemifumarate | ||
|---|---|---|---|---|
| CAS Number | 130580-02-8 | Molecular Weight | 444.45300 | |
| Density | N/A | Boiling Point | 456.3ºC at 760mmHg | |
| Molecular Formula | C23H25FN2O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 229.8ºC | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of Eplivanserin hemifumarateEplivanserin (SR-46349) hemifumarate is a potent, selective and orally active 5-HT2A receptor antagonist, with an IC50 of 5.8 nM in rat cortical membrane, and a Kd of 1.14 nM. Eplivanserin hemifumarate displays >20-fold selectivity more selective for 5-HT2A than 5-HT2B and 5-HT2C[1][2]. |
| Name | Eplivanserin Fumarate |
|---|---|
| Synonym | More Synonyms |
| Description | Eplivanserin (SR-46349) hemifumarate is a potent, selective and orally active 5-HT2A receptor antagonist, with an IC50 of 5.8 nM in rat cortical membrane, and a Kd of 1.14 nM. Eplivanserin hemifumarate displays >20-fold selectivity more selective for 5-HT2A than 5-HT2B and 5-HT2C[1][2]. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A Receptor:5.8 nM (IC50) 5-HT2C Receptor:120 nM (IC50) |
| In Vitro | Eplivanserin hemifumarate (SR 46349B) shows weak inhibition on other 5-HT kinases, with IC50s of 0.12 μM (Pig cortex 5-HT1C), 14 μM (Rat hippocampus 5-HT1A), and 16 μM (Rat stnatum 5-HT1B, Ox caudate nucleus 5-HT1D). Eplivanserin also has inhibitory effects on rat cortex adrenergic α1 and α2, rat whole brain histammine H1, Na+ channel, and rat striatum dopamine D1 and D2, with IC50s of 3.4 μM, 1.0 μM, 5.0 μM, 39 μM, 9 μM and 28 μM, respectively[1]. |
| In Vivo | Eplivanserin hemifumarate (SR 46349B) inhibits 5-HT2 receptor binding of [3H]ketanserin with an ED50 of 0.087 mg/kg after i.p. injection, and 0.097 mg/kg after p.o administration in mice[1]. SR 46349B (0.25-1 mg/kg; i.p.) blocks Cocaine-evoked hyperactivity following repeated Cocaine treatment in rats[2]. |
| References |
| Boiling Point | 456.3ºC at 760mmHg |
|---|---|
| Molecular Formula | C23H25FN2O6 |
| Molecular Weight | 444.45300 |
| Flash Point | 229.8ºC |
| Exact Mass | 444.17000 |
| PSA | 119.66000 |
| LogP | 3.23880 |
| Vapour Pressure | 1.63E-08mmHg at 25°C |
| InChIKey | RNLKLYQQDLHHBH-WCIJYLBISA-N |
| SMILES | CN(C)CCON=C(C=Cc1ccc(O)cc1)c1ccccc1F.CN(C)CCON=C(C=Cc1ccc(O)cc1)c1ccccc1F.O=C(O)C=CC(=O)O |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H410 |
| Precautionary Statements | P273-P501 |
| RIDADR | UN 3077 9 / PGIII |
|
Nest building is impaired in the Ts65Dn mouse model of Down syndrome and rescued by blocking 5HT2a receptors.
Neurobiol. Learn. Mem. 116 , 162-71, (2014) Down syndrome (DS) has an incidence of about 1/700 births, and is therefore the most common cause of cognitive and behavioral impairments in children. Recent studies on mouse models of DS indicate tha... |
|
|
Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats.
Pharmacol. Rep. 57(1) , 35-46, (2005) Previous studies have indicated a role of serotonin (5-HT)2 receptors in modulation of the behavioral effects of cocaine. In the present study, the efficacy of SR 46349B (a 5-HT(2A) receptor antagonis... |
|
|
Activation of slowly conducting medullary raphe-spinal neurons, including serotonergic neurons, increases cutaneous sympathetic vasomotor discharge in rabbit.
Am. J. Physiol. Regul. Integr. Comp. Physiol. 288(4) , R909-18, (2005) Neurons in the rostral medullary raphe/parapyramidal region regulate cutaneous sympathetic nerve discharge. Using focal electrical stimulation at different dorsoventral raphe/parapyramidal sites in an... |
| Eplivanserin hemifumarate |
| (Z,E)-1-(2-Fluorophenyl)-3-(4-hydroxyphenyl)-2-propen-1-one O-[2-(dimethylamino)ethyl]oxime fumarate (2:1) |
| TRANS-2'-FLUORO-4-HYDROXYCHALCONE O-[(Z)-2-(DIMETHYLAMINO)ETHYL]OXIME--FUMARIC ACID (2:1) |
| SR-46349 hemifumarate salt |