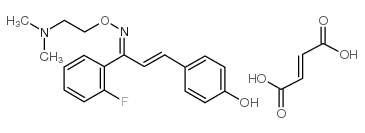
130580-02-8
| Name | Eplivanserin Fumarate |
|---|---|
| Synonyms |
Eplivanserin hemifumarate
(Z,E)-1-(2-Fluorophenyl)-3-(4-hydroxyphenyl)-2-propen-1-one O-[2-(dimethylamino)ethyl]oxime fumarate (2:1) TRANS-2'-FLUORO-4-HYDROXYCHALCONE O-[(Z)-2-(DIMETHYLAMINO)ETHYL]OXIME--FUMARIC ACID (2:1) SR-46349 hemifumarate salt |
| Description | Eplivanserin (SR-46349) hemifumarate is a potent, selective and orally active 5-HT2A receptor antagonist, with an IC50 of 5.8 nM in rat cortical membrane, and a Kd of 1.14 nM. Eplivanserin hemifumarate displays >20-fold selectivity more selective for 5-HT2A than 5-HT2B and 5-HT2C[1][2]. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A Receptor:5.8 nM (IC50) 5-HT2C Receptor:120 nM (IC50) |
| In Vitro | Eplivanserin hemifumarate (SR 46349B) shows weak inhibition on other 5-HT kinases, with IC50s of 0.12 μM (Pig cortex 5-HT1C), 14 μM (Rat hippocampus 5-HT1A), and 16 μM (Rat stnatum 5-HT1B, Ox caudate nucleus 5-HT1D). Eplivanserin also has inhibitory effects on rat cortex adrenergic α1 and α2, rat whole brain histammine H1, Na+ channel, and rat striatum dopamine D1 and D2, with IC50s of 3.4 μM, 1.0 μM, 5.0 μM, 39 μM, 9 μM and 28 μM, respectively[1]. |
| In Vivo | Eplivanserin hemifumarate (SR 46349B) inhibits 5-HT2 receptor binding of [3H]ketanserin with an ED50 of 0.087 mg/kg after i.p. injection, and 0.097 mg/kg after p.o administration in mice[1]. SR 46349B (0.25-1 mg/kg; i.p.) blocks Cocaine-evoked hyperactivity following repeated Cocaine treatment in rats[2]. |
| References |
| Boiling Point | 456.3ºC at 760mmHg |
|---|---|
| Molecular Formula | C23H25FN2O6 |
| Molecular Weight | 444.45300 |
| Flash Point | 229.8ºC |
| Exact Mass | 444.17000 |
| PSA | 119.66000 |
| LogP | 3.23880 |
| Vapour Pressure | 1.63E-08mmHg at 25°C |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H410 |
| Precautionary Statements | P273-P501 |
| RIDADR | UN 3077 9 / PGIII |