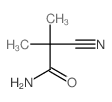
173334-58-2
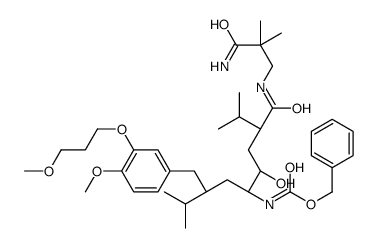
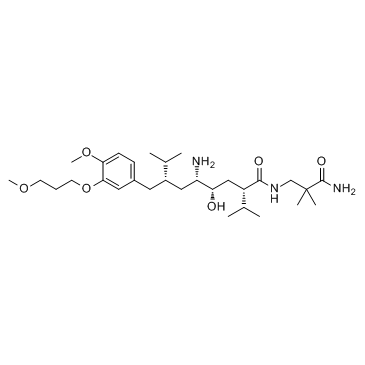
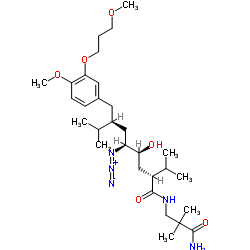
| Name | Aliskiren hemifumarate |
|---|---|
| Synonyms |
CGP60536B
Aliskiren fumarate tekturna (2S,4S,5S,7S)-5-Amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide (2E)-2-butenedioate (2:1) Aliskiren hemifumarate (2E)-But-2-endisäure--(2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methyl-2-(1-methylethyl)nonanamid(1:2) Benzeneoctanamide, δ-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-γ-hydroxy-4-methoxy-3-(3-methoxypropoxy)-α,ζ-bis(1-methylethyl)-, (αS,γS,δS,ζS)-, (2E)-2-butenedioate (2:1) (salt) CGP 60536 (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methyl-2-(1-methylethyl)nonanamide (2E)-but-2-enedioate (2:1) (salt) Benzeneoctanamide, δ-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-γ-hydroxy-4-methoxy-3-(3-methoxypropoxy)-α,ζ-bis(1-methylethyl)-, (αS,γS,δS,ζS)-, (2E)-2-butenedioate (2:1
 ) (salt) acide (2E)-but-2-ènedioïque - (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-diméthyl-3-oxopropyl)-4-hydroxy-7-[4-méthoxy-3-(3-méthoxypropoxy)benzyl]-8-méthyl-2-(1-méthyléthyl)nonanamide (1:2) Rasilez SPP 100 (2S,4S,5S,7S)-5-Amino-N-(2-carbamoyl-2,2-dimethylethyl)-4-hydroxy-7-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-8-methyl-2-(propan-2-yl)nonanamide hemifumarate benzeneoctanamide, δ-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-γ-hydroxy-4-methoxy-3-(3-methoxypropoxy)-α,ζ-bis(1-methylethyl)-, (αS,γS,δS,ζS)-, (2E)-2-butenedioate (2:1 Aliskirenhemifumarate Aliskiren (hemifumarate) |
| Description | Aliskiren hemifumarate(CGP 60536) is a direct renin inhibitor with IC50 of 1.5 nM.IC50 value: 1.5 nM [1]Target: reninin vitro: Aliskiren hemifumarate appears to bind to both the hydrophobic S1/S3-binding pocket and to a large, distinct subpocket that extends from the S3-binding site towards the hydrophobic core of renin. Oral bioavailability of Aliskiren hemifumarate is 2.4% in rats, 16% in marmosets and about 2.5% in humans [2].in vivo: Aliskiren hemifumarate (< 10 mg/kg, oral) inhibits plasma renin activity and lowers blood pressure in sodium-depleted marmosets[3].Once-daily oral treatment with Aliskiren hemifumarate lowers blood pressure effectively, with a safety and tolerability profile, in patients with mild-to-moderate hypertension[4]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 72-75?C |
|---|---|
| Molecular Formula | C64H110N6O16 |
| Molecular Weight | 1219.59000 |
| Exact Mass | 1218.80000 |
| PSA | 366.86000 |
| LogP | 9.88180 |
| Vapour Pressure | 1.59E-23mmHg at 25°C |
| Storage condition | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| Hazard Codes | Xi |
|---|
|
~97% 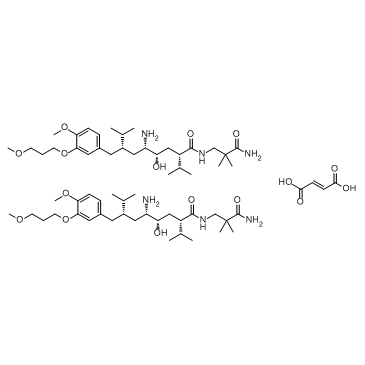
173334-58-2 |
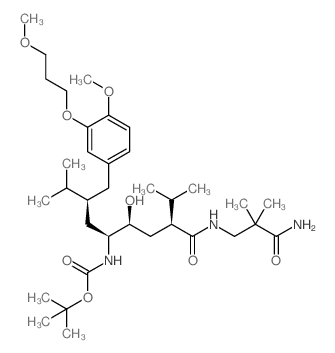
| Literature: CHEMO IBERICA, S. A.; CARCONE, Luca; MAGRONE, Domenico; BARRECA, Giuseppe; RASPARINI, Marcello; LI MING, Huan Patent: WO2013/14191 A1, 2013 ; Location in patent: Page/Page column 19; 20 ; |
|
~86% 
173334-58-2 |
| Literature: CHEMO IBERICA, S. A.; CARCONE, Luca; MAGRONE, Domenico; BARRECA, Giuseppe; RASPARINI, Marcello; LI MING, Huan Patent: WO2013/14191 A1, 2013 ; Location in patent: Page/Page column 19 ; |
|
~81% 
173334-58-2 |
| Literature: JUBILANT LIFE SCIENCES LIMITED; BISWAS, Sujay; SRIMURUGAN, Sankareswaran; KUMAR, Anjul; PANDA, Atulya, Kumar; JAMSHAD, Danish; MASAND, Mukesh; BISWAS, Bidyut; BANSAL, Vikas; GUPTA, Ashish, Kumar; VIR, Dharam Patent: WO2013/61224 A1, 2013 ; Location in patent: Page/Page column 25 ; |
|
~% 
173334-58-2 |
| Literature: WO2003/103653 A1, ; Page 196 ; WO 03/103653 A1 |
|
~% 
173334-58-2 |
| Literature: WO2012/52829 A1, ; Page/Page column 16-17 ; |
|
~% 
173334-58-2 |
| Literature: WO2011/148392 A1, ; |
|
~% 
173334-58-2 |
| Literature: WO2011/148392 A1, ; |
|
~% 
173334-58-2 |
| Literature: WO2012/52829 A1, ; |
|
~% 
173334-58-2 |
| Literature: WO2011/148392 A1, ; |
| Precursor 10 | |
|---|---|
| DownStream 1 | |