Ro 0437626
Modify Date: 2025-08-23 19:07:34
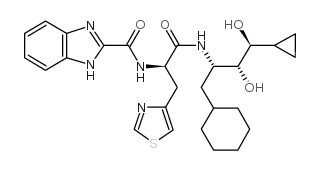
Ro 0437626 structure
|
Common Name | Ro 0437626 | ||
|---|---|---|---|---|
| CAS Number | 134362-79-1 | Molecular Weight | 525.66300 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H35N5O4S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Ro 0437626Ro 0437626 is a selective purinergic (P2X1) receptor antagonist (IC50 = 3 μM), but shows low affinity for P2X2, P2X3 and P2X2/3 receptors (IC50 > 100 μM)[1]. |
| Name | N-[(2R)-1-[[(2S,3R,4S)-1-cyclohexyl-4-cyclopropyl-3,4-dihydroxybutan-2-yl]amino]-1-oxo-3-(1,3-thiazol-4-yl)propan-2-yl]-1H-benzimidazole-2-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | Ro 0437626 is a selective purinergic (P2X1) receptor antagonist (IC50 = 3 μM), but shows low affinity for P2X2, P2X3 and P2X2/3 receptors (IC50 > 100 μM)[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3 μM (P2X1 receptor) |
| In Vitro | Ro 0437626 reduces PMA-evoked Ca2+entry with 6.8 ± 4.7% of control[2]. |
| In Vivo | Ro 0437626 (1 and 10 μmol/kg; i.v.) causes a reduction in postinfusion isovolumetric contractions[3]. Animal Model: Female rat (urethane-anaesthetized)[3] Dosage: 1 and 10 μmol/kg Administration: I.v. Result: Caused a reduction in postinfusion isovolumetric contractions. |
| References |
| Molecular Formula | C27H35N5O4S |
|---|---|
| Molecular Weight | 525.66300 |
| Exact Mass | 525.24100 |
| PSA | 168.47000 |
| LogP | 3.72930 |
| InChIKey | JHRSGCIIDRWHCD-UARRHKHWSA-N |
| SMILES | O=C(NC(Cc1cscn1)C(=O)NC(CC1CCCCC1)C(O)C(O)C1CC1)c1nc2ccccc2[nH]1 |
| Cortistatin 14 |