1-(5-Methyl-2-thienyl)ethanone
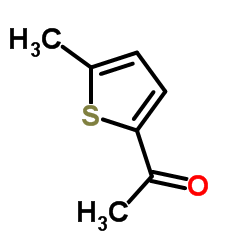
1-(5-Methyl-2-thienyl)ethanone structure
|
Common Name | 1-(5-Methyl-2-thienyl)ethanone | ||
|---|---|---|---|---|
| CAS Number | 13679-74-8 | Molecular Weight | 140.203 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 234.9±20.0 °C at 760 mmHg | |
| Molecular Formula | C7H8OS | Melting Point | 24-28 °C(lit.) | |
| MSDS | USA | Flash Point | 95.8±21.8 °C | |
Use of 1-(5-Methyl-2-thienyl)ethanone2-Acetyl-5-methylthiophene is a constituent of coffee aroma. |
| Name | 2-Acetyl-5-methylthiophene |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 234.9±20.0 °C at 760 mmHg |
| Melting Point | 24-28 °C(lit.) |
| Molecular Formula | C7H8OS |
| Molecular Weight | 140.203 |
| Flash Point | 95.8±21.8 °C |
| Exact Mass | 140.029587 |
| PSA | 45.31000 |
| LogP | 1.71 |
| Vapour Pressure | 0.1±0.5 mmHg at 25°C |
| Index of Refraction | 1.536 |
| InChIKey | YOSDTJYMDAEEAZ-UHFFFAOYSA-N |
| SMILES | CC(=O)c1ccc(C)s1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful;Xi: Irritant; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S36/37-S22 |
| RIDADR | UN 3335 9 |
| WGK Germany | 3 |
| RTECS | OB4972000 |
| HS Code | 2934999090 |
|
~97% 
1-(5-Methyl-2-t... CAS#:13679-74-8 |
| Literature: Altenhoener, Kai; Lamm, Jan-Hendrik; Mattay, Jochen European Journal of Organic Chemistry, 2010 , # 31 p. 6033 - 6037 |
|
~67% 
1-(5-Methyl-2-t... CAS#:13679-74-8 |
| Literature: SUMITOMO SEIKA CHEMICALS CO., LTD. Patent: EP1695972 A1, 2006 ; Location in patent: Page/Page column 7 ; |
| Precursor 3 | |
|---|---|
| DownStream 9 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Acylation studies in the thiophene and furan series. IV. Strong inorganic oxyacids as catalysts. Hartough HD and Kosak AI.
J. Am. Chem. Soc. 69(12) , 3093-3096, (1947)
|
|
|
Palladium-catalysed direct 3-or 4-arylation of thiophene derivatives using aryl bromides. Dong JJ, et al.
Tetrahedron 50(23) , 2778-2781, (2009)
|
|
|
Efficient guaiazulene and chamazulene syntheses involving [6+4] cycloadditions. Mukherjee D, et al.
J. Am. Chem. Soc. 1010(1) , 251-252, (1979)
|
| Thiophene, 2-acetyl-5-methyl- |
| 1-(5-Methyl-2-thienyl)ethanone |
| 1-(5-Methyl-2-thienyl)ethanone |
| 2-Acetyl-5-methylthiophene |
| T5SJ BV1 E1 |
| Ethanone, 1-(5-methyl-2-thienyl)- |
| 1-(5-methylthien-2-yl)ethanone |
| Methyl 5-methyl-2-thienyl ketone |
| 1-(5-Methyl-2-thienyl)ethan-1-one |
| 1-(5-methylthiophen-2-yl)ethanone |
| MFCD00014529 |
| EINECS 237-181-2 |
| Ketone, methyl 5-methyl-2-thienyl |


 CAS#:4282-31-9
CAS#:4282-31-9 CAS#:1918-79-2
CAS#:1918-79-2 CAS#:40323-88-4
CAS#:40323-88-4 CAS#:326-72-7
CAS#:326-72-7 CAS#:4066-41-5
CAS#:4066-41-5 CAS#:31555-59-6
CAS#:31555-59-6![2-[3,5-bis(5-methylthiophen-2-yl)phenyl]-5-methylthiophene structure](https://image.chemsrc.com/caspic/053/13792-96-6.png) CAS#:13792-96-6
CAS#:13792-96-6 CAS#:36901-17-4
CAS#:36901-17-4 CAS#:70624-30-5
CAS#:70624-30-5
