CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
BZ8225000
-
CHEMICAL NAME :
-
Anisole, p-allyl-
-
CAS REGISTRY NUMBER :
-
140-67-0
-
BEILSTEIN REFERENCE NO. :
-
1099454
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C10-H12-O
-
MOLECULAR WEIGHT :
-
148.22
-
WISWESSER LINE NOTATION :
-
1U2R DO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1230 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1030 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1250 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - coma
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1260 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
97 gm/kg/1Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Liver - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
111 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Liver - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
140 mg/kg/22D-I
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Lungs, Thorax, or Respiration - tumors Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
195 gm/kg/1Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Liver - tumors Liver - angiosarcoma
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
80 mg/kg
-
REFERENCE :
-
CRNGDP Carcinogenesis (London). (Oxford Univ. Press, Pinkhill House, Southfield Road, Eynsham, Oxford OX8 1JJ, UK) V.1- 1980- Volume(issue)/page/year: 5,1613,1984 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X5058 No. of Facilities: 668 (estimated) No. of Industries: 2 No. of Occupations: 6 No. of Employees: 9128 (estimated) No. of Female Employees: 6777 (estimated)
|




 CAS#:104-92-7
CAS#:104-92-7 CAS#:106-95-6
CAS#:106-95-6 CAS#:623-12-1
CAS#:623-12-1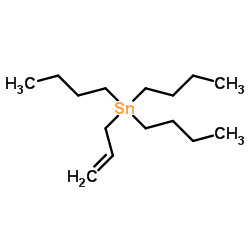 CAS#:24850-33-7
CAS#:24850-33-7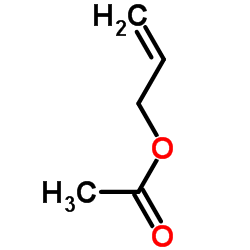 CAS#:591-87-7
CAS#:591-87-7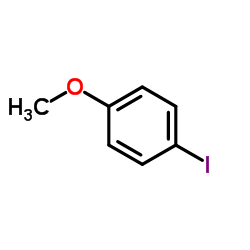 CAS#:696-62-8
CAS#:696-62-8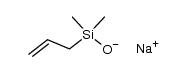 CAS#:1092390-58-3
CAS#:1092390-58-3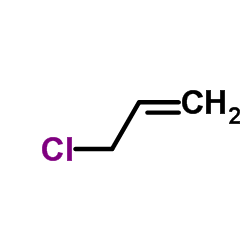 CAS#:107-05-1
CAS#:107-05-1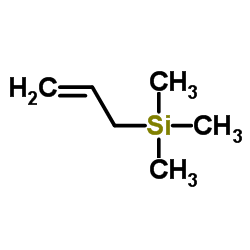 CAS#:762-72-1
CAS#:762-72-1 CAS#:15183-74-1
CAS#:15183-74-1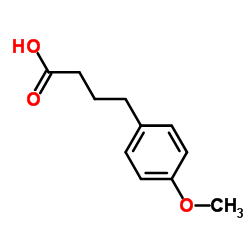 CAS#:4521-28-2
CAS#:4521-28-2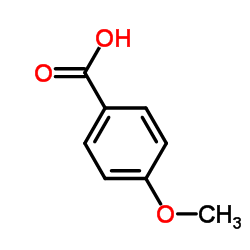 CAS#:100-09-4
CAS#:100-09-4 CAS#:104-01-8
CAS#:104-01-8 CAS#:104-45-0
CAS#:104-45-0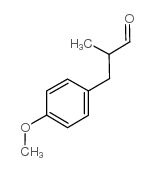 CAS#:5462-06-6
CAS#:5462-06-6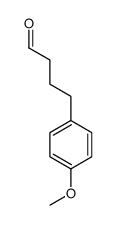 CAS#:56047-51-9
CAS#:56047-51-9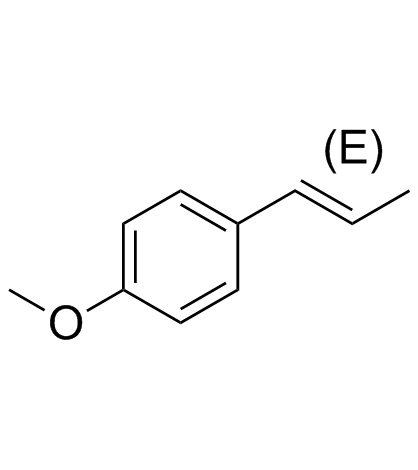 CAS#:4180-23-8
CAS#:4180-23-8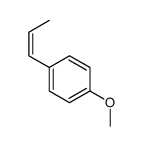 CAS#:25679-28-1
CAS#:25679-28-1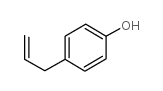 CAS#:501-92-8
CAS#:501-92-8 CAS#:20637-08-5
CAS#:20637-08-5
