p-Boronobenzoic acid
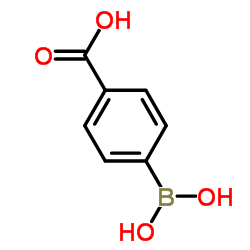
p-Boronobenzoic acid structure
|
Common Name | p-Boronobenzoic acid | ||
|---|---|---|---|---|
| CAS Number | 14047-29-1 | Molecular Weight | 165.94 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 406.4±47.0 °C at 760 mmHg | |
| Molecular Formula | C7H7BO4 | Melting Point | 220 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 199.6±29.3 °C | |
Use of p-Boronobenzoic acid4-Boronobenzoic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-Carboxyphenylboronic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Boronobenzoic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 406.4±47.0 °C at 760 mmHg |
| Melting Point | 220 °C (dec.)(lit.) |
| Molecular Formula | C7H7BO4 |
| Molecular Weight | 165.94 |
| Flash Point | 199.6±29.3 °C |
| Exact Mass | 166.043747 |
| PSA | 77.76000 |
| LogP | 1.27 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.585 |
| InChIKey | SIAVMDKGVRXFAX-UHFFFAOYSA-N |
| SMILES | O=C(O)c1ccc(B(O)O)cc1 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | CY8925000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
|
Highly efficient aerobic oxidative hydroxylation of arylboronic acids: photoredox catalysis using visible light.
Angew. Chem. Int. Ed. Engl. 3rd ed., 51 , 784-788, (2012)
|
|
|
A new access to 3-substituted-1(2H)-isoquinolone by tandem palladium-catalyzed intramolecular aminocarbonylation annulation.
Org. Biomol. Chem. 13th ed., 10 , 2683-2691, (2012) An original tribromide derivative based, palladium-catalyzed synthesis of 3-substituted-1(2H)-isoquinolone is described based on a regioselective Suzuki-Miyaura C-C coupling on o-halo-(2,2-dihalovinyl... |
|
|
Synthesis of new trisulfonated calix[4]arenes functionalized at the upper rim, and their complexation with the trimethyllysine epigenetic mark.
Org. Lett. 6th ed., 14 , 1512-1515, (2012) A synthetic route to produce a new family of trisulfonated calix[4]arenes bearing a single group, selectively introduced, that lines the binding pocket is reported. Ten examples, including new sulfona... |
| 4-(Dihydroxyboryl)benzoic acid |
| Benzoic acid, 4-borono- |
| 4-Carboxybenzeneboronic Acid |
| 4-Boronobenzoic acid |
| MFCD00151801 |
| 4-Carboxyphenylboronic acid |
| p-Carboxybenzeneboronic acid |
| p-Boronobenzoic acid |
| 4-(dihydroxyboranyl)benzoic acid |
 CAS#:5720-05-8
CAS#:5720-05-8![8-(4-carboxyphenyl)-4-methyl-2,6-dioxohexahydro-[1,3,2]oxazaborolo[2,3-b][1,3,2]oxazaborol-4-ium-8-uide Structure](https://image.chemsrc.com/caspic/024/1072960-67-8.png) CAS#:1072960-67-8
CAS#:1072960-67-8 CAS#:7722-64-7
CAS#:7722-64-7 CAS#:126747-14-6
CAS#:126747-14-6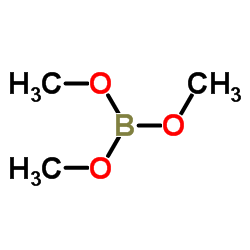 CAS#:121-43-7
CAS#:121-43-7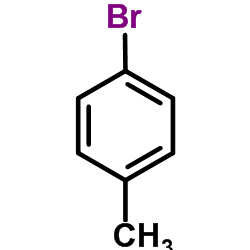 CAS#:106-38-7
CAS#:106-38-7 CAS#:124-38-9
CAS#:124-38-9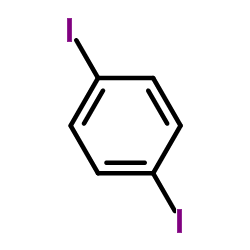 CAS#:624-38-4
CAS#:624-38-4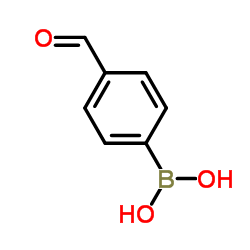 CAS#:87199-17-5
CAS#:87199-17-5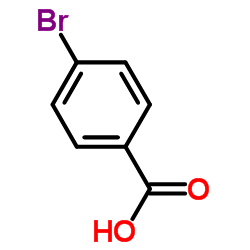 CAS#:586-76-5
CAS#:586-76-5![[4-(Diethylcarbamoyl)phenyl]boronic acid structure](https://image.chemsrc.com/caspic/312/389621-80-1.png) CAS#:389621-80-1
CAS#:389621-80-1![2'-METHYL-[1,1'-BIPHENYL]-4-CARBOXYLIC ACID structure](https://image.chemsrc.com/caspic/035/5748-43-6.png) CAS#:5748-43-6
CAS#:5748-43-6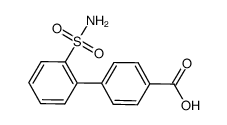 CAS#:352615-90-8
CAS#:352615-90-8![[4-(Isopropylcarbamoyl)phenyl]boronic acid structure](https://image.chemsrc.com/caspic/122/397843-67-3.png) CAS#:397843-67-3
CAS#:397843-67-3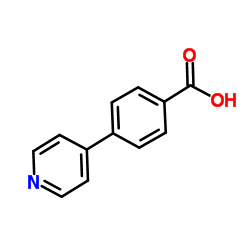 CAS#:4385-76-6
CAS#:4385-76-6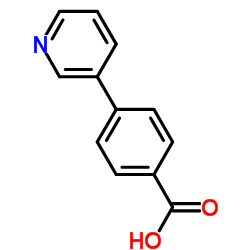 CAS#:4385-75-5
CAS#:4385-75-5 CAS#:3808-93-3
CAS#:3808-93-3 CAS#:332154-57-1
CAS#:332154-57-1 CAS#:330793-45-8
CAS#:330793-45-8![[4-(Cyclopropylcarbamoyl)phenyl]boronic acid structure](https://image.chemsrc.com/caspic/340/515140-26-8.png) CAS#:515140-26-8
CAS#:515140-26-8
