WEHI-539
Modify Date: 2024-01-13 17:36:20
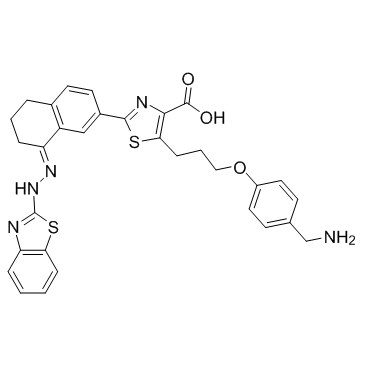
WEHI-539 structure
|
Common Name | WEHI-539 | ||
|---|---|---|---|---|
| CAS Number | 1431866-33-9 | Molecular Weight | 583.724 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 827.6±75.0 °C at 760 mmHg | |
| Molecular Formula | C31H29N5O3S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 454.3±37.1 °C | |
Use of WEHI-539WEHI-539 is a selective inhibitor of Bcl-XL with IC50 of 1.1 nM. |
| Name | 5-[3-[4-(aminomethyl)phenoxy]propyl]-2-[(8E)-8-(1,3-benzothiazol-2-ylhydrazinylidene)-6,7-dihydro-5H-naphthalen-2-yl]-1,3-thiazole-4-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | WEHI-539 is a selective inhibitor of Bcl-XL with IC50 of 1.1 nM. |
|---|---|
| Related Catalog | |
| Target |
Bcl-xL:1.1 nM (IC50) |
| In Vitro | WEHI-539 is a selective inhibitor of Bcl-XL. WEHI-539 augments Carboplatin induced caspase 3/7 activity, PARP cleavage and annexin V labelling. WEHI-539 as a single agent causes noticeable PARP cleavage in Ovcar-4 (5 μM in Ovcar-4.) and Ovsaho (1 μM in Ovsaho) cells[2]. |
| Cell Assay | Ovcar-8, Ovcar-3, Ovcar-4 and Ovsaho cells are grown in the RPMI, Igrov-1, Cov-362 and Cov-318 cells are grown in DMEM and Fuov-1 cells are grown in DMEM/F-12 nutrient mixture. ABT-737, ABT-199 and WEHI-539 (Medchem Express, NJ, USA), are prepared as a 20 mM solution in DMSO. For cell growth assays, cells are plated in 96 wells plate (5,000 cells/well for all cell lines except Ovcar-8 which is plated at a density of 2,500 cells/well). The next day, cells are treated with drugs. After 72 h the culture medium is removed and the cells are fixed with 100 μL of cold 10 % Trichloroacetic acid (TCA), incubated on ice for 30 min and stained with 0.4 % sulforhodamine B (SRB). The data are analysed by using Graphpad Prism 4 software. Non-linear regression is used to fit a four parameters Hill equation. For drug combinations studies the cells are exposed simultaneously to a range of concentrations of carboplatin combined with fixed concentration of BH3 mimetics that is expected from the single agent studies to cause 5 % growth inhibition: ABT-737, 1 μM in Ovcar-8, Ovcar-3 and Igrov-1, 2 μM in Ovcar-4 and Ovsaho and 6 μM in Cov-362; ABT-199, 1 μM in Ovcar-4, 2 μM in Ovcar-3, Igrov-1, Cov-362 and Ovsaho and 3 μM in Ovcar-8; WEHI-539, 0.2 μM in Igrov-1, 0.3 μM in Ovcar-8, 1 μM in Ovcar-3 and Ovsaho, 3.1 μM in Cov-362 and 5 μM in Ovcar-4. Surviving cell number is assessed by SRB staining. A combination index (CI) is calculated[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 827.6±75.0 °C at 760 mmHg |
| Molecular Formula | C31H29N5O3S2 |
| Molecular Weight | 583.724 |
| Flash Point | 454.3±37.1 °C |
| Exact Mass | 583.171204 |
| PSA | 179.20000 |
| LogP | 6.85 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.737 |
| Storage condition | 2-8℃ |
| WEHI-539 |
| 5-(3-(4-(Aminomethyl)phenoxy)propyl)-2-(8-(2-(benzo[d]thiazol-2-yl)hydrazono)-5,6,7,8-tetrahydronaphthalen-2-yl)thiazole-4-carboxylic acid |
| CS-1330 |
| WEHI-539||WEHI 539 |
| 4-Thiazolecarboxylic acid, 5-[3-[4-(aminomethyl)phenoxy]propyl]-2-[(8E)-8-[2-(2-benzothiazolyl)hydrazinylidene]-5,6,7,8-tetrahydro-2-naphthalenyl]- |
| 5-{3-[4-(Aminomethyl)phenoxy]propyl}-2-[(8E)-8-(1,3-benzothiazol-2-ylhydrazono)-5,6,7,8-tetrahydro-2-naphthalenyl]-1,3-thiazole-4-carboxylic acid |