5,6-Dimethoxyindole
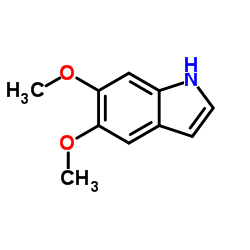
5,6-Dimethoxyindole structure
|
Common Name | 5,6-Dimethoxyindole | ||
|---|---|---|---|---|
| CAS Number | 14430-23-0 | Molecular Weight | 177.200 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 320.3±22.0 °C at 760 mmHg | |
| Molecular Formula | C10H11NO2 | Melting Point | 154-157 °C(lit.) | |
| MSDS | USA | Flash Point | 117.4±12.6 °C | |
| Name | 5,6-dimethoxyindole |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 320.3±22.0 °C at 760 mmHg |
| Melting Point | 154-157 °C(lit.) |
| Molecular Formula | C10H11NO2 |
| Molecular Weight | 177.200 |
| Flash Point | 117.4±12.6 °C |
| Exact Mass | 177.078979 |
| PSA | 34.25000 |
| LogP | 1.82 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 6 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
[Monoamine oxidase inhibitors. I. Synthesis of N-cyclopropyltryptamines].
Farmaco. Sci. 35(9) , 785-90, (1980) The synthesis of two new N-cyclopropyltryptamines is described. By treating 5,6-dimethoxyindole with oxalyl chloride and N-benzylcyclopropylamine, N-benzyl-N-cyclopropyl-5,6-dimethoxyindole-3-glyoxala... |
|
|
Cross-polarization dynamics and spin diffusion in some aromatic compounds.
Solid State Nucl. Magn. Reson. 3(3) , 121-35, (1994) The inversion-recovery cross-polarization (IRCP) magic-angle spinning experiment has been applied to study the 13C-1H cross-polarization dynamics of protonated aromatic carbons in ferrocene, 5,6-dimet... |
|
|
Structural characterization of two isomeric dimethoxyindoles of biological interest, using high- and low-energy collisional spectroscopy.
Rapid Commun. Mass Spectrom. 3(12) , 413-6, (1989) It is not possible to distinguish isomers of biologically important dimethoxyindoles using electron-ionization mass spectra, but they may be distinguished by collisionally activated dissociation. In p... |
| 1H-Indole, 5,6-dimethoxy- |
| 5,6-Dimethoxyindole |
| MFCD00005675 |
| 5,6-Dimethoxy-1H-indole |
| EINECS 238-402-5 |
 CAS#:65907-71-3
CAS#:65907-71-3 CAS#:106103-20-2
CAS#:106103-20-2 CAS#:16551-84-1
CAS#:16551-84-1 CAS#:106103-12-2
CAS#:106103-12-2 CAS#:4460-86-0
CAS#:4460-86-0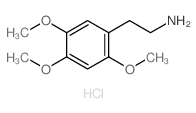 CAS#:3166-78-7
CAS#:3166-78-7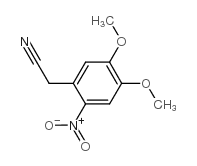 CAS#:17354-04-0
CAS#:17354-04-0 CAS#:93-17-4
CAS#:93-17-4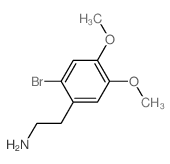 CAS#:63375-81-5
CAS#:63375-81-5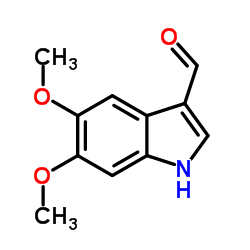 CAS#:142769-27-5
CAS#:142769-27-5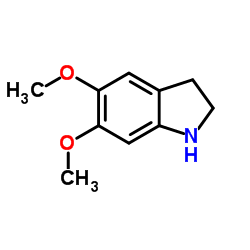 CAS#:15937-07-2
CAS#:15937-07-2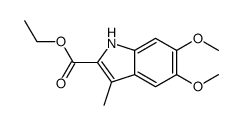 CAS#:16381-44-5
CAS#:16381-44-5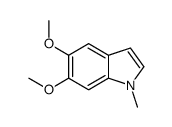 CAS#:80639-40-3
CAS#:80639-40-3 CAS#:57330-45-7
CAS#:57330-45-7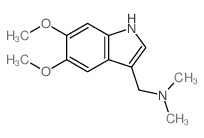 CAS#:5446-82-2
CAS#:5446-82-2
