Candesartan cilexetil

Candesartan cilexetil structure
|
Common Name | Candesartan cilexetil | ||
|---|---|---|---|---|
| CAS Number | 145040-37-5 | Molecular Weight | 610.660 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 843.3±75.0 °C at 760 mmHg | |
| Molecular Formula | C33H34N6O6 | Melting Point | 168-170?C | |
| MSDS | Chinese USA | Flash Point | 463.8±37.1 °C | |
| Symbol |


GHS08, GHS09 |
Signal Word | Warning | |
Use of Candesartan cilexetilCandesartan Cilexetil (TCV-116) is an angiotensin II receptor antagonist used mainly for the treatment of hypertension.Target: Type-1 angiotensin II receptorCandesartan is generally well tolerated and significantly reduced cardiovascular deaths and hospital admissions for heart failure. Ejection fraction or treatment at baseline did not alter these effects [1]. In rats, TCV-116 inhibited the pressor responses to Ang I, Ang II, and Ang III without an effect on the bradykinin (BK)-induced depressor response. In SHR, the antihypertensive effect of TCV-116 (10 mg/kg) was larger than the maximum antihypertensive effect of enalapril and was not intensified by combination with enalapril. TCV-116 is more effective than enalapril in reducing blood pressure in SHR and 1K, 1C-HR, and that the BK- and/or prostaglandin-potentiating effect of enalapril contributes little to its antihypertensive mechanism in SHR [2]. |
| Name | Candesartan cilexetil |
|---|---|
| Synonym | More Synonyms |
| Description | Candesartan Cilexetil (TCV-116) is an angiotensin II receptor antagonist used mainly for the treatment of hypertension.Target: Type-1 angiotensin II receptorCandesartan is generally well tolerated and significantly reduced cardiovascular deaths and hospital admissions for heart failure. Ejection fraction or treatment at baseline did not alter these effects [1]. In rats, TCV-116 inhibited the pressor responses to Ang I, Ang II, and Ang III without an effect on the bradykinin (BK)-induced depressor response. In SHR, the antihypertensive effect of TCV-116 (10 mg/kg) was larger than the maximum antihypertensive effect of enalapril and was not intensified by combination with enalapril. TCV-116 is more effective than enalapril in reducing blood pressure in SHR and 1K, 1C-HR, and that the BK- and/or prostaglandin-potentiating effect of enalapril contributes little to its antihypertensive mechanism in SHR [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 843.3±75.0 °C at 760 mmHg |
| Melting Point | 168-170?C |
| Molecular Formula | C33H34N6O6 |
| Molecular Weight | 610.660 |
| Flash Point | 463.8±37.1 °C |
| Exact Mass | 610.253967 |
| PSA | 143.34000 |
| LogP | 7.79 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.667 |
| Storage condition | -20°C Freezer |
| Symbol |


GHS08, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H361-H400 |
| Precautionary Statements | P280 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 4 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Development and characterization of mixed niosomes for oral delivery using candesartan cilexetil as a model poorly water-soluble drug.
AAPS PharmSciTech 16(1) , 108-17, (2015) The aim of this study was to prepare candesartan cilexetil-loaded niosomes and mixed niosomes to enhance the aqueous solubility of the drug, thus improving its oral bioavailability. The formulations w... |
|
|
Use of compounded dispersing media for extemporaneous pediatric syrups with candesartan cilexetil and valsartan.
Acta. Pharm. 64(4) , 463-74, (2014) Available tablets or capsules for adults are often used to prepare extemporaneously formulated medicines appropriate for children. The most acceptable drug forms in pediatric population are oral liqui... |
|
|
Hepatic, intestinal, renal, and plasma hydrolysis of prodrugs in human, cynomolgus monkey, dog, and rat: implications for in vitro-in vivo extrapolation of clearance of prodrugs.
Drug Metab. Dispos. 42(9) , 1522-31, (2014) Hydrolysis plays an important role in metabolic activation of prodrugs. In the current study, species and in vitro system differences in hepatic and extrahepatic hydrolysis were investigated for 11 pr... |
| 1-{[(Cyclohexyloxy)carbonyl]oxy}ethyl 2-ethoxy-1-{[2'-(1H-tetrazol-5-yl)-4-biphenylyl]methyl}-1H-benzimidazole-7-carboxylate |
| Candesartan cilexetil |
| 1-{[(cyclohexyloxy)carbonyl]oxy}ethyl 2-ethoxy-1-{[2'-(2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate |
| 2-Ethoxy-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1H-benzimidazole-7-carboxylic Acid 1-[[(Cyclohexyloxy)carbonyl]oxy]ethyl Ester |
| UNII:R85M2X0D68 |
| MFCD00871371 |
| 1-{[(Cyclohexyloxy)carbonyl]oxy}ethyl 2-ethoxy-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate |
| 1H-Benzimidazole-7-carboxylic acid, 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-, 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester |
| Candesartan (Cilexetil) |
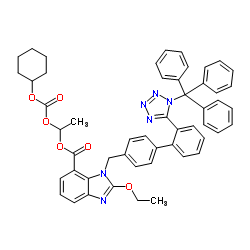 CAS#:170791-09-0
CAS#:170791-09-0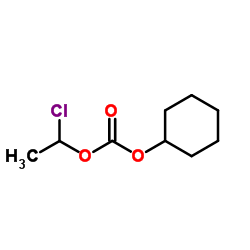 CAS#:99464-83-2
CAS#:99464-83-2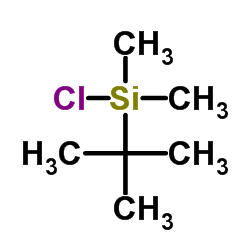 CAS#:18162-48-6
CAS#:18162-48-6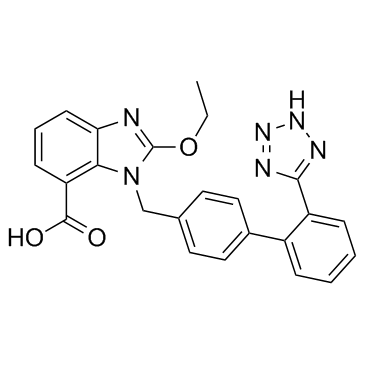 CAS#:139481-59-7
CAS#:139481-59-7![1-(cyclohexyloxycarbonyloxy)ethyl 1-{(2'-cyanobiphenyl-4-yl)methyl}-2-ethoxy-1H-benzo[d]imidazole-7-carboxylate Structure](https://image.chemsrc.com/caspic/490/632322-62-4.png) CAS#:632322-62-4
CAS#:632322-62-4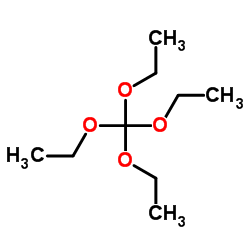 CAS#:78-09-1
CAS#:78-09-1![(+/-)-1-[[(cyclohexyloxy)carbonyl]oxy]ethyl 3-amino-2-[[[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]amino]benzoate Structure](https://image.chemsrc.com/caspic/071/1236156-65-2.png) CAS#:1236156-65-2
CAS#:1236156-65-2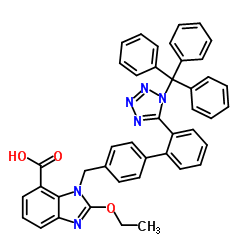 CAS#:139481-72-4
CAS#:139481-72-4 CAS#:869631-11-8
CAS#:869631-11-8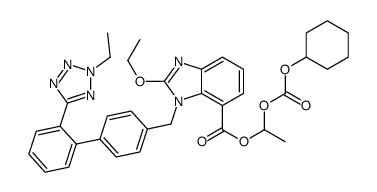 CAS#:914613-36-8
CAS#:914613-36-8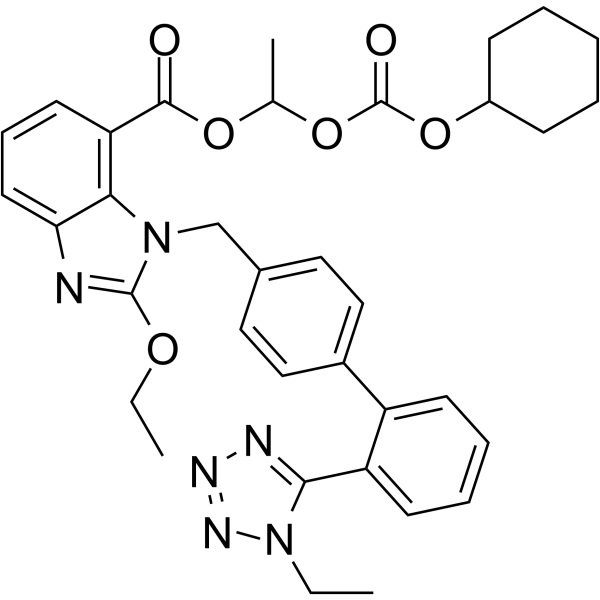 CAS#:914613-35-7
CAS#:914613-35-7
