Benserazide hydrochloride
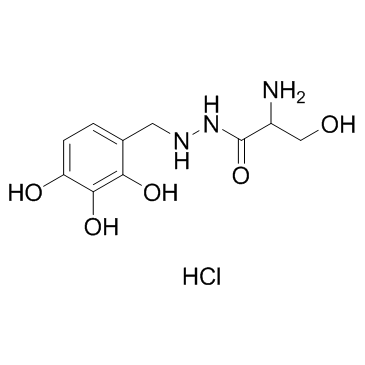
Benserazide hydrochloride structure
|
Common Name | Benserazide hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 14919-77-8 | Molecular Weight | 293.704 | |
| Density | N/A | Boiling Point | 574.2ºC at 760 mmHg | |
| Molecular Formula | C10H16ClN3O5 | Melting Point | 146°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Benserazide hydrochlorideBenserazide Hydrochloride is a peripherally-acting aromatic L-amino acid decarboxylase (AAAD) or DOPA decarboxylase inhibitor.Target: DOPA decarboxylaseBenserazide is commonly used for Parkinson's disease in combination with L-DOPA as a peripheral aromatic L-amino acid decarboxylase (AADC) inhibitor. Administration of 5, 10 and 50 mg/kg benserazide to 6-OHDA-lesioned rats showed an identical increase in exogenous L-DOPA-derived extracellular DA levels, the time to reach the peak DA levels were significantly prolonged by benserazide dose-dependently. The AADC activity in the denervated striatal tissues showed a significant decrease by 10 mg/kg and 50 mg/kg benserazide. These results suggest that benserazide reduces the central AADC activity in the striatum of rats with nigrostriatal denervation, which leads to changes in the metabolism of exogenous L-DOPA [1]. |
| Name | benserazide hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Benserazide Hydrochloride is a peripherally-acting aromatic L-amino acid decarboxylase (AAAD) or DOPA decarboxylase inhibitor.Target: DOPA decarboxylaseBenserazide is commonly used for Parkinson's disease in combination with L-DOPA as a peripheral aromatic L-amino acid decarboxylase (AADC) inhibitor. Administration of 5, 10 and 50 mg/kg benserazide to 6-OHDA-lesioned rats showed an identical increase in exogenous L-DOPA-derived extracellular DA levels, the time to reach the peak DA levels were significantly prolonged by benserazide dose-dependently. The AADC activity in the denervated striatal tissues showed a significant decrease by 10 mg/kg and 50 mg/kg benserazide. These results suggest that benserazide reduces the central AADC activity in the striatum of rats with nigrostriatal denervation, which leads to changes in the metabolism of exogenous L-DOPA [1]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 574.2ºC at 760 mmHg |
|---|---|
| Melting Point | 146°C |
| Molecular Formula | C10H16ClN3O5 |
| Molecular Weight | 293.704 |
| Exact Mass | 293.077850 |
| PSA | 148.07000 |
| LogP | 0.52790 |
| InChIKey | ULFCBIUXQQYDEI-UHFFFAOYSA-N |
| SMILES | Cl.NC(CO)C(=O)NNCc1ccc(O)c(O)c1O |
| Storage condition | -20°C Freezer |
| Water Solubility | H2O: 10 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | VT9632300 |
| HS Code | 2928000090 |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Adenosine A1 receptor stimulation reduces D1 receptor-mediated GABAergic transmission from striato-nigral terminals and attenuates l-DOPA-induced dyskinesia in dopamine-denervated mice
Exp. Neurol. 261 , 733-43, (2014) γ-Aminobutyric acid A receptor (GABAAR)-mediated postsynaptic currents were recorded in brain slices from substantia nigra pars reticulate neurons. The selective adenosine A1 receptor (A1R) antagonist... |
|
|
Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons.
Brain 137(Pt 8) , 2287-302, (2014) Dopamine midbrain neurons within the substantia nigra are particularly prone to degeneration in Parkinson's disease. Their selective loss causes the major motor symptoms of Parkinson's disease, but th... |
|
|
L-DOPA-induced dyskinesia in a rat model of Parkinson's disease is associated with the fluctuational release of norepinephrine in the sensorimotor striatum.
J. Neurosci. Res. 92(12) , 1733-45, (2014) L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia (LID) is the most common complication of standard L-DOPA therapy for Parkinson's disease experienced by most parkinsonian patients. LID is asso... |
| DL-Serine 2-(2,3,4-trihydroxybenzyl)hydrazide hydrochloride |
| Ro 4-4602 |
| BENSERAZIDE HYDROCHLORIDE EP2000 |
| EINECS 238-991-9 |
| Benserazide HCl |
| BENPARIZDE |
| Benserazide hydrochloride |
| Benserazide |
| BENSERAZIDE HYDROCHLORIDE PERIPHERAL D |
| 2-Amino-3-hydroxy-N'-(2,3,4-trihydroxybenzyl)propanehydrazide hydrochloride (1:1) (non-preferred name) |
| MFCD00078571 |
| (S)-2-Amino-3-hydroxy-N'-(2,3,4-trihydroxybenzyl)propanehydrazide hydrochloride |
| Ro 4-4602/001 |
| 2-Amino-3-hydroxy-N'-(2,3,4-trihydroxybenzyl)propanehydrazide hydrochloride |
| Benserazide (hydrochloride) |

