Carmustine
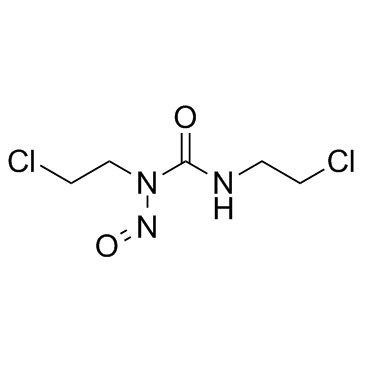
Carmustine structure
|
Common Name | Carmustine | ||
|---|---|---|---|---|
| CAS Number | 154-93-8 | Molecular Weight | 214.050 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 404ºC | |
| Molecular Formula | C5H9Cl2N3O2 | Melting Point | 30 °C(lit.) | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of CarmustineCarmustine is an antitumor chemotherapeutic agent, which works by akylating DNA and RNA. |
| Name | carmustine |
|---|---|
| Synonym | More Synonyms |
| Description | Carmustine is an antitumor chemotherapeutic agent, which works by akylating DNA and RNA. |
|---|---|
| Related Catalog | |
| Target |
DNA Alkylator[1] |
| In Vitro | Carmustine is an antitumor chemotherapeutic agent. Carmustine (8, 80, and 800 µM) decreases N-acetyltransferase (NAT) activities for 2-aminofluorene (AF) and p-aminobenzoic acid (PABA) in rat glial tumor cytosol and intact cells. In rat glial tumor cells, the DNA-AF adduct increases, and carmustine decreases the formation of DNA-AF adduct[1]. |
| In Vivo | Carmustine (BCNU; 25 mg/kg, i.p.) causes higher levels of the rhe ratio of liver weight to body weight and plasma conjugated bilirubin, and lower biliary flow, oxidised glutation levels (GSSG) and reduced glutation (GSH)/GSSG values compared with control rats[2]. |
| Kinase Assay | The determination of Acetyl-CoAdependent N-acetylation of 2-aminofluorene (AF) and p-aminobenzoic acid (PABA) are performed. Incubation mixtures in the assay system consists of a total volume of 90 μL: glial tumor cells cytosols, diluted as required, in 50 μL of lysis buffer (20 mM Tris/HCl, pH 7.5, 1 mM DTT and 1 mM EDTA), 20 μL of an Acetyl-CoA recycling mixture of 50 mM Tris-HCl (pH7.5), 0.2 mM EDTA, 2 mM DTT, 15 mM acetylcamitine, 2U/mL carnitine acetyltransferase, and AF or PABA at specific concentrations. The reactions are started by addition of 20 μL of Acetyl-CoA. The control reactions have 20 μL distilled water in place of Acetyl-CoA. For the single point activity measurements, the final concentration of AF or PABA is 0.1 mM and AcCoA is 0.5 mM. The reaction mixtures with or without specific concentrations of Carmustine and lomustine are incubated at 37°C for 10 min and stopped with 50 μL of 20% trichloroacetic acid for the PABA reactions, and 100 μL of acetonitrile for the AF reactions. All of the reactions (experiments and controls) are run in triplicate[1]. |
| Animal Admin | Rats[2] Individual rats are weighted prior to enter the study; their weights are recorded, and they are randomLy assigned to four groups. Group I (saline group); This group consists of 12 rats. These rats are injected with 2 mL/kg of saline intraperitoneally (IP) 48 h before the study, being included by the study 48 h later. Group II (corn oil group) consists of 15 rats. These rats are injected with 2 mL/kg of corn oil (vehicle) IP 48 h before the study. Group III (Carmustine group) consists of 16 rats. These rats are injected with 1 mL per day of saline IP, administered at the same hour of the day as a single-dose for 3 days. Twelve hours after the first dose of saline, corn oil 2 mL/kg + Carmustine 25 mg/kg IP are injected, and the rats are included in the study 48 h after the administration of corn oil + Carmustine. Group IV (trimetazidine group) consists of 12 rats. These rats are injected with 2.5 mg/kg per day of trimetazidine (TMZ) IP, administered at the same hour of the day as a single-dose for 3 days. 12 h after the first dose of TMZ, corn oil 2 mL/kg + Carmustine 25 mg/kg IP are injected, and the rats are included in the study 48 h after the administration of corn oil + Carmustine[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 404ºC |
| Melting Point | 30 °C(lit.) |
| Molecular Formula | C5H9Cl2N3O2 |
| Molecular Weight | 214.050 |
| Exact Mass | 213.007187 |
| PSA | 61.77000 |
| LogP | 1.30 |
| Index of Refraction | 1.549 |
| Water Solubility | <0.1 g/100 mL at 18 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H350-H360 |
| Precautionary Statements | P201-P264-P301 + P310-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+:Verytoxic; |
| Risk Phrases | R45;R46;R60;R61;R28 |
| Safety Phrases | S53-S22-S36/37/39-S45 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | YS2625000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2924199090 |
|
~97% 
Carmustine CAS#:154-93-8 |
| Literature: Celaries, Benoit; Parkanyi, Cyril Synthesis, 2006 , # 14 p. 2371 - 2375 |
|
~% 
Carmustine CAS#:154-93-8 |
| Literature: Synthesis, , # 11 p. 1027 - 1029 |
| Precursor 3 | |
|---|---|
| DownStream 8 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system.
Nat. Commun. 6 , 6761, (2015) How the brain's antioxidant defenses adapt to changing demand is incompletely understood. Here we show that synaptic activity is coupled, via the NMDA receptor (NMDAR), to control of the glutathione a... |
|
|
Inhibitors of hydroperoxide metabolism enhance ascorbate-induced cytotoxicity.
Free Radic. Res. 47(3) , 154-63, (2013) Pharmacological ascorbate, via its oxidation, has been proposed as a pro-drug for the delivery of H(2)O(2) to tumors. Pharmacological ascorbate decreases clonogenic survival of pancreatic cancer cells... |
|
|
Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma.
Int. J. Pharm. 495 , 972-80, (2015) In this work, multifunctional lipid nanocapsules (M-LNC) were designed to combine the activity of the cytotoxic drug paclitaxel (PTX) with the immunostimulant CpG. This nanosystem, consisting of modif... |
| 1,3-bis(2-Chlorethyl)-1-nitrosourea |
| Bicnu |
| Becenun |
| Carmustine |
| BCNU |
| EINECS 205-838-2 |
| Urea, N,N'-bis(2-chloroethyl)-N-nitroso- |
| MFCD00057706 |
| 1,3-Bis(2-chloroethyl)-1-nitrosourea |
| Carustine |
| 1,3-Bis(2-chloroethyl)-1-nitrosourea BCNU |
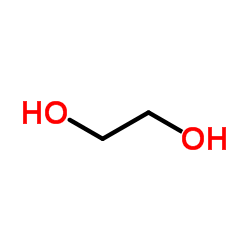 CAS#:107-21-1
CAS#:107-21-1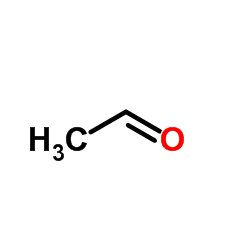 CAS#:75-07-0
CAS#:75-07-0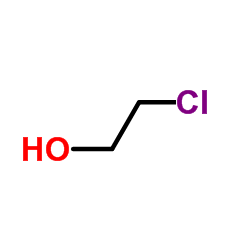 CAS#:107-07-3
CAS#:107-07-3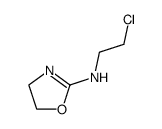 CAS#:71353-16-7
CAS#:71353-16-7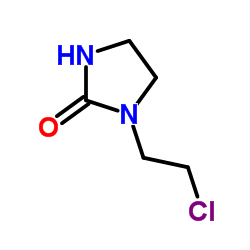 CAS#:2387-20-4
CAS#:2387-20-4 CAS#:64-17-5
CAS#:64-17-5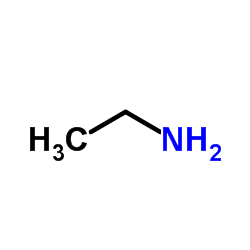 CAS#:75-04-7
CAS#:75-04-7