TBB
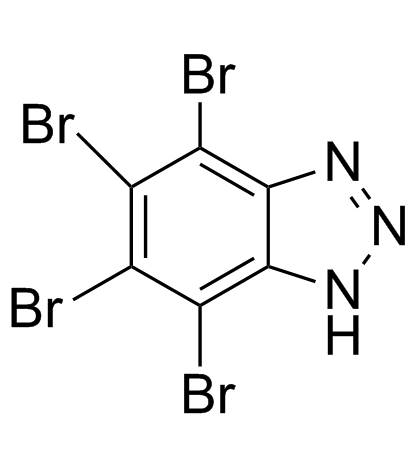
TBB structure
|
Common Name | TBB | ||
|---|---|---|---|---|
| CAS Number | 17374-26-4 | Molecular Weight | 434.708 | |
| Density | 2.8±0.1 g/cm3 | Boiling Point | 552.5±45.0 °C at 760 mmHg | |
| Molecular Formula | C6HBr4N3 | Melting Point | 262-266ºC | |
| MSDS | USA | Flash Point | 288.0±28.7 °C | |
Use of TBBTBB is a cell-permeable and ATP-competitive CK2 inhibitor with an IC50 of 0.15 μM for rat liver CK2. |
| Name | 4,5,6,7-Tetrabromo-1H-benzotriazole |
|---|---|
| Synonym | More Synonyms |
| Description | TBB is a cell-permeable and ATP-competitive CK2 inhibitor with an IC50 of 0.15 μM for rat liver CK2. |
|---|---|
| Related Catalog | |
| Target |
CK2:0.15 μM (IC50, Human CK2) PIM1:1.04 μM (IC50) PIM2:4.3 μM (IC50) PIM3:0.86 μM (IC50) HIPK2:5.3 μM (IC50) HIPK3:4.9 μM (IC50) DYRK1a:4.36 μM (IC50) DYRK2:0.99 μM (IC50) DYRK3:5.3 μM (IC50) PKD1:5.9 μM (IC50) CDK2:14 μM (IC50) |
| In Vitro | Investigation of the inhibitory power of TBB with a panel of 33 protein kinases shows highest potency for CK2 (casein kinase 2) (human CK2: IC50=1.6 μM at 100 μM ATP). TBB also inhibits three other kinases with less potency: CDK2 (IC50=15.6 μM), phosphorylase kinase (IC50=8.7 μM) and glycogen synthase kinase 3β (GSK3β) (IC50=11.2 μM). All other kinases tested have IC50 values 50-fold greater than that for CK2[1]. The viability of the androgen insensitive PC-3 cells may be diminished by TBB (60 μM TBB) acting either alone or combined with anticancer agents CPT or TRAIL when a proper time schedule of the administration is applied. The time schedule-dependent activity of TBB does not come from its effect on apoptosis in PC-3 cells[2]. TBB is an ATP/GTP competitive inhibitor of protein kinase casein kinase-2 (CK2), has been examined against a panel of 33 protein kinases, either Ser/Thr- or Tyr-specific. In the presence of 10 μM TBB (and 100 μM ATP) only CK2 is drastically inhibited (>85%) whereas three kinases (phosphorylase kinase, glycogen synthase kinase 3L and cyclin-dependent kinase 2/cyclin A) underwent moderate inhibition, with IC50 values one-two orders of magnitude higher than CK2 (IC50=0.9 μM). TBB also inhibits endogenous CK2 in cultured Jurkat cells[3]. |
| In Vivo | The extent of retinal neovascularization in a mouse OIR model is reduced by approximately 60% after treatment with TBB (6 days at 60 mg/kg per day)[4]. |
| Cell Assay | PC-3 or HeLa cells are cultured routinely in RPMI-1640 and DMEM media, respectively, which are supplemented with 10% FBS, Penicillin (100 U/mL) and Streptomycin (100 μg/mL) at 37°C in a humidified atmosphere of 5% CO2. Cells are seeded at 5×104 cells/well (PC-3) or 2×104 (HeLa) in 24-wells plates and cultured for 72 h. TBB (final concentration 60 μM), CPT (final concentration 5.8 nM), 2-deoxyglucose (2-DG; final concentration 0.5 mM) or TRAIL (final concentration 13.3 ng/mL) are added to the medium individually or in a combination and the cells are cultured for additional time, indicated on each figure. After treatment, the medium with the agent is removed and 500 μL of MTT mixture (0.5 mg/mL for PC-3 and 5.0 mg/mL for HeLa cells in medium without phenol red) is added to each well and incubated for an additional 1 h at 37°C. The formazan crystals are diluted in 250 μL of DMSO. The absorbance is measured at 570 nm[2]. |
| Animal Admin | Mice[4] The heterozygous C57BL/6J mice are used. Emodin and TBB are injected intraperitoneally in volumes of 50 μL or less per mouse at doses of 15 to 30 mg/kg body weight, twice daily, starting from day 11. Control mice are injected with PEG-Tween vehicle alone. |
| References |
| Density | 2.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 552.5±45.0 °C at 760 mmHg |
| Melting Point | 262-266ºC |
| Molecular Formula | C6HBr4N3 |
| Molecular Weight | 434.708 |
| Flash Point | 288.0±28.7 °C |
| Exact Mass | 430.690369 |
| PSA | 41.57000 |
| LogP | 3.96 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.801 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~% 
TBB CAS#:17374-26-4 |
| Literature: Journal of the American Chemical Society, , vol. 79, p. 4395,4399 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
ATG16L1 phosphorylation is oppositely regulated by CSNK2/casein kinase 2 and PPP1/protein phosphatase 1 which determines the fate of cardiomyocytes during hypoxia/reoxygenation.
Autophagy 11 , 1308-25, (2015) Recent studies have shown that the phosphorylation and dephosphorylation of ULK1 and ATG13 are related to autophagy activity. Although ATG16L1 is absolutely required for autophagy induction by affecti... |
|
|
Casein Kinase 2 Negatively Regulates Abscisic Acid-Activated SnRK2s in the Core Abscisic Acid-Signaling Module.
Mol. Plant 8 , 709-21, (2015) SnRK2 kinases, PP2C phosphatases and the PYR/PYL/RCAR receptors constitute the core abscisic acid (ABA) signaling module that is thought to contain all of the intrinsic properties to self-regulate the... |
|
|
Identification of malaria parasite-infected red blood cell surface aptamers by inertial microfluidic SELEX (I-SELEX).
Sci. Rep. 5 , 11347, (2015) Plasmodium falciparum malaria parasites invade and remodel human red blood cells (RBCs) by trafficking parasite-synthesized proteins to the RBC surface. While these proteins mediate interactions with ... |
| 4,5,6,7-Tetrabromo-1H-benzotriazole |
| 4,5,6,7-Tetrabromobenzotriazole |
| 4,5,6,7-tetrabromo-2H-benzotriazole |
| 4,5,6,7-Tetrabromo-2-azabenzimidazole 4,5,6,7-Tetrabromobenzotriazole |
| 1H-1,2,3-Benzotriazole, 4,5,6,7-tetrabromo- |
| 4,5,6,7-tetrabromo-2-azabenzimidazole |
| 4,5,6,7-Tetrabromo-2-azabenzimidazole,4,5,6,7-Tetrabromobenzotriazole |
| TBB |
| NSC 231634 |
| 1H-Benzotriazole,4,5,6,7-tetrabromo- |