SR 142948A
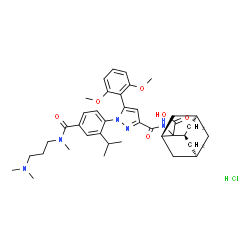
SR 142948A structure
|
Common Name | SR 142948A | ||
|---|---|---|---|---|
| CAS Number | 184162-21-8 | Molecular Weight | 722.313 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C39H51N5O6 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of SR 142948AA potent, selective and orally active neurotensin receptor antagonist with IC50 of 1.19 nM; antagonizes neurotensin-induced inositol monophosphate formation in HT 29 cells with IC50 of 3.9 nM, inhibits the turning behavior induced by neurotensin in mice, completely antagonizes neurotensin-evoked acetylcholine release in the rat striatum at 0.1 mg/kg; also blocks both hypothermia and analgesia induced by i.c.v. injection of neurotensin injection into the ventral tegmental area. |
| Name | SR 142948A |
|---|---|
| Synonym | More Synonyms |
| Description | A potent, selective and orally active neurotensin receptor antagonist with IC50 of 1.19 nM; antagonizes neurotensin-induced inositol monophosphate formation in HT 29 cells with IC50 of 3.9 nM, inhibits the turning behavior induced by neurotensin in mice, completely antagonizes neurotensin-evoked acetylcholine release in the rat striatum at 0.1 mg/kg; also blocks both hypothermia and analgesia induced by i.c.v. injection of neurotensin injection into the ventral tegmental area. |
|---|---|
| References | References 1. Gully D, et al. J Pharmacol Exp Ther. 1997 Feb;280(2):802-12. 2. Betancur C, et al. Eur J Pharmacol. 1998 Feb 5;343(1):67-77. 3. Schaeffer P, et al. J Cardiovasc Pharmacol. 1998 Apr;31(4):545-50. View Related Products by Target Neurotensin Receptor |
| Molecular Formula | C39H51N5O6 |
|---|---|
| Molecular Weight | 722.313 |
| Exact Mass | 721.360596 |
| Storage condition | 2-8°C |
| RIDADR | NONH for all modes of transport |
|---|
|
Biochemical and pharmacological activities of SR 142948A, a new potent neurotensin receptor antagonist.
J. Pharmacol. Exp. Ther. 280(2) , 802-12, (1997) SR 142948A, 2-[[5-(2,6-dimethoxyphenyl)-1-(4-(N-(3-dimethylaminopropyl)-N-methylc arbamoyl)-2-isopropylphenyl)-1H-pyrazole3-carbonyl]amino] adamantane-2-carboxylic acid, hydrochloride, a new and extre... |
|
|
Neurotensin receptor antagonist SR 142948A alters Fos expression and extrapyramidal side effect profile of typical and atypical antipsychotic drugs.
Biol. Psychiatry 29(12) , 2200-7, (2004) Antipsychotic drugs (APDs) have previously been shown to alter Fos expression in a regionally specific manner. Increases in Fos expression in the nucleus accumbens (NAcc) are common to all clinically ... |
|
|
Neurotensin signaling induces intracellular alkalinization and interleukin-8 expression in human pancreatic cancer cells.
Mol. Oncol. 3(3) , 204-13, (2009) Pancreatic adenocarcinomas express neurotensin receptors in up to 90% of cases, however, their role in tumor biology and as a drug target is not clear. In the present study, a stable neurotensin (NT) ... |
| MFCD03106502 |
| SR 142948 hydrochloride |
| (1R,5R)-2-({[5-(2,6-Dimethoxyphenyl)-1-(4-{[3-(dimethylamino)propyl](methyl)carbamoyl}-2-isopropylphenyl)-1H-pyrazol-3-yl]carbonyl}amino)-2-adamantanecarboxylic acid hydrochloride (1:1) |
| 2-[[5-(2,6-dimethoxyphenyl)-1-(4-(N-(3-dimethylaminopropyl)-N-methylcarbamoyl)-2-isopropylphenyl)-1H-pyrazole3-carbonyl]amino] adamantane-2-carboxylic acid hydrochloride |
| SR 142948A |
| Tricyclo[3.3.1.13,7]decane-2-carboxylic acid, 2-[[[5-(2,6-dimethoxyphenyl)-1-[4-[[[3-(dimethylamino)propyl]methylamino]carbonyl]-2-(1-methylethyl)phenyl]-1H-pyrazol-3-yl]carbonyl]amino]-, (1R,5R)-, hydrochloride (1:1) |