Sangivamycin
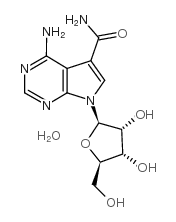
Sangivamycin structure
|
Common Name | Sangivamycin | ||
|---|---|---|---|---|
| CAS Number | 18417-89-5 | Molecular Weight | 309.28 | |
| Density | N/A | Boiling Point | 880.6ºC at 760 mmHg | |
| Molecular Formula | C12H15N5O5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 486.4ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of SangivamycinSangivamycin (NSC 65346), a nucleoside analog, is a potent inhibitor of protein kinase C (PKC) with an Ki of 10 μM. Sangivamycin has potent antiproliferative activity against a variety of human cancers[1][2]. |
| Name | sangivamycin |
|---|---|
| Synonym | More Synonyms |
| Description | Sangivamycin (NSC 65346), a nucleoside analog, is a potent inhibitor of protein kinase C (PKC) with an Ki of 10 μM. Sangivamycin has potent antiproliferative activity against a variety of human cancers[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Sangivamycin has differential antitumor effects in drug-sensitive MCF7/wild type (WT) cells, causing growth arrest, and in multidrug-resistant MCF7/adriamycin-resistant (ADR) human breast carcinoma cells, causing massive apoptotic cell death[2]. Sangivamycin (0.3 μM; 0-72 hours), shows almost maximal cytocidal (for MCF7/ADR) or cytostatic (for MCF7/WT) effects[2]. Sangivamycin activates caspases in MCF7/ADR cells. Upon exposure of MCF7/ADR cells to Sangivamycin (0.3 μM;), a vast amount of cleavage of lamin A to a 28-kDa fragment is detected within 48 hours[2]. |
| References |
| Boiling Point | 880.6ºC at 760 mmHg |
|---|---|
| Molecular Formula | C12H15N5O5 |
| Molecular Weight | 309.28 |
| Flash Point | 486.4ºC |
| Exact Mass | 327.11800 |
| PSA | 178.97000 |
| Vapour Pressure | 3.3E-33mmHg at 25°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H310-H330 |
| Precautionary Statements | P260-P264-P280-P284-P302 + P350-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+ |
| Risk Phrases | 26/27/28 |
| RIDADR | UN 2811 6.1/PG 2 |
| RTECS | UY9355000 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
Synthesis and in vitro antitumor activity of some amino-deoxy 7-hexofuranosylpyrrolo[2,3-d]pyrimidines.
Carbohydr. Res. 308(3-4) , 319-28, (1998) 7-(6-amino-6-deoxy-beta-D-glucofuranosyl)-5-cyanopyrrolo[2,3 -d]pyrimidine (22) and 7-(3-amino-methyl-3-deoxy-beta-D-allofuranosyl)-5- cyanopyrrolo[2,3-d]pyrimidine (28) were synthesized by sequential... |
|
|
Kinetics and localization of the phosphorylation of rhodopsin by protein kinase C.
J. Biol. Chem. 270(12) , 6710-7, (1995) Protein kinase C isolated from retina catalyzes the stoichiometric phosphorylation of bovine rhodopsin. Enzymological studies using receptor in rod outer segment membranes stripped of peripheral prote... |
|
|
Synthesis of pyrrolo[2,1-f][1,2,4]triazine C-nucleosides. Isosteres of sangivamycin, tubercidin, and toyocamycin.
Carbohydr. Res. 331(1) , 77-82, (2001) Syntheses of pyrrolo[2,1-f][1,2,4]triazine C-nucleosides are reported. Treatment of pyranulose glycoside with aminoguanidine in acetic acid gave the corresponding semicarbazone in 96% yield. The ring ... |
| 7-Carboxamido-7-deazaadenosine |
| 7-DEAZA-7-CARBAMOYLADENOSINE |
| 7-DEAZAADENOSINE-7-CARBOXAMIDE |
| SANGIVAMYCIN |
![7H-Pyrrolo[2,3-d]pyrimidine-5-carboxamide,4-amino-7-(5-deoxy-b-D-ribofuranosyl)- structure](https://www.chemsrc.com/caspic/110/65562-56-3.png) CAS#:65562-56-3
CAS#:65562-56-3