Dioscin
Modify Date: 2024-01-02 08:17:32
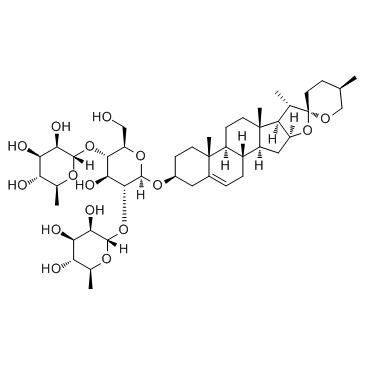
Dioscin structure
|
Common Name | Dioscin | ||
|---|---|---|---|---|
| CAS Number | 19057-60-4 | Molecular Weight | 869.044 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C45H72O16 | Melting Point | 294-296 ℃ | |
| MSDS | N/A | Flash Point | N/A | |
Use of DioscinDioscin(CCRIS 4123; Collettiside III) is a natural steroid saponin derived from several plants, showing potent anti-cancer effect against a variety of tumor cell lines. IC50 value:Target: Anticancer agentin vitro: dioscin (1, 2 and 4 μmol/L) could significantly inhibit the viability of LNCaP cells in a time- and concentration-dependent manner. Flow cytometry revealed that the apoptosis rate was increased after treatment of LNCaP cells with dioscin for 24 h, indicating that apoptosis was an important mechanism by which dioscin inhibited cancer [1]. dioscin abrogated AKT phosphorylation, which subsequently impaired RANKL-induced nuclear factor-kappaB (NF-κB) signaling pathway and inhibited NFATc1 transcriptional activity. Moreover, in vivo studies further verified the bone protection activity of dioscin in osteolytic animal model [2]. Dioscin reduced cell death and lactate dehydrogenase (LDH) release in cells subjected to I/R. I/R induced apoptosis and cytochrome c release from mitochondria to the cytosol and this was prevented by dioscin. In support, dioscin decreased Bax but increased Bcl-2 mRNA expression. Dioscin prevented I/R induced dissipation of ΔΨm [3]. |
| Name | dioscin |
|---|---|
| Synonym | More Synonyms |
| Description | Dioscin(CCRIS 4123; Collettiside III) is a natural steroid saponin derived from several plants, showing potent anti-cancer effect against a variety of tumor cell lines. IC50 value:Target: Anticancer agentin vitro: dioscin (1, 2 and 4 μmol/L) could significantly inhibit the viability of LNCaP cells in a time- and concentration-dependent manner. Flow cytometry revealed that the apoptosis rate was increased after treatment of LNCaP cells with dioscin for 24 h, indicating that apoptosis was an important mechanism by which dioscin inhibited cancer [1]. dioscin abrogated AKT phosphorylation, which subsequently impaired RANKL-induced nuclear factor-kappaB (NF-κB) signaling pathway and inhibited NFATc1 transcriptional activity. Moreover, in vivo studies further verified the bone protection activity of dioscin in osteolytic animal model [2]. Dioscin reduced cell death and lactate dehydrogenase (LDH) release in cells subjected to I/R. I/R induced apoptosis and cytochrome c release from mitochondria to the cytosol and this was prevented by dioscin. In support, dioscin decreased Bax but increased Bcl-2 mRNA expression. Dioscin prevented I/R induced dissipation of ΔΨm [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 294-296 ℃ |
| Molecular Formula | C45H72O16 |
| Molecular Weight | 869.044 |
| Exact Mass | 868.482056 |
| PSA | 235.68000 |
| LogP | 7.24 |
| Index of Refraction | 1.614 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| (3β,25R)-Spirost-5-en-3-yl 6-deoxy-α-L-mannopyranosyl-(1->2)-[6-deoxy-α-L-mannopyranosyl-(1->4)]-β-D-glucopyranoside |
| collettisideiii |
| (25R)-Spirost-5-en-3b-yl O-6-Deoxy-a-L-mannopyranosyl-(1®2)-O-[6-deoxy-a-L-mannopyranosyl-(1®4)]-b-D-glucopyranoside |
| β-D-Glucopyranoside, (3β,25R)-spirost-5-en-3-yl O-6-deoxy-α-L-mannopyranosyl-(1->2)-O-[6-deoxy-α-L-mannopyranosyl-(1->4)]- |
| Dioscin |
| polyphyllin III |
| Diosgenin Bis-a-L-rhamnopyranosyl-(1®2 And 1®4)-b-D-Glucopyranoside |

