Epieriocalyxin A
Modify Date: 2024-01-20 17:40:03
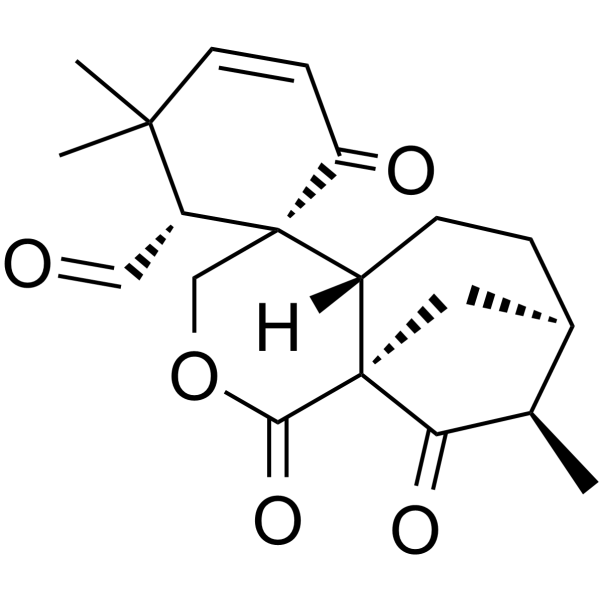
Epieriocalyxin A structure
|
Common Name | Epieriocalyxin A | ||
|---|---|---|---|---|
| CAS Number | 191545-24-1 | Molecular Weight | 344.402 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 563.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H24O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 248.4±30.2 °C | |
Use of Epieriocalyxin Aepi-Eriocalyxin A (Epieriocalyxin A), a diterpenoid isolated from Isodon eriocalyx, induces colon cancer apoptosis. epi-Eriocalyxin A also inhibits ERK1/2 and JNK activation, which suppresses Bcl-2 expression[1]. |
| Name | (1S,1'S,2R,6'S,9'R,10'R)-3,3,10'-Trimethyl-2',6,11'-trioxo-3'-oxaspiro[cyclohexane-1,5'-tricyclo[7.2.1.01,6]dodecane]-4-ene-2-carbaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | epi-Eriocalyxin A (Epieriocalyxin A), a diterpenoid isolated from Isodon eriocalyx, induces colon cancer apoptosis. epi-Eriocalyxin A also inhibits ERK1/2 and JNK activation, which suppresses Bcl-2 expression[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 563.4±50.0 °C at 760 mmHg |
| Molecular Formula | C20H24O5 |
| Molecular Weight | 344.402 |
| Flash Point | 248.4±30.2 °C |
| Exact Mass | 344.162384 |
| PSA | 77.51000 |
| LogP | 1.69 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.563 |
| InChIKey | NTFGUZQAIRRLSN-ULQCDAFVSA-N |
| SMILES | CC1C(=O)C23CC1CCC2C1(COC3=O)C(=O)C=CC(C)(C)C1C=O |
| Hazard Codes | Xi |
|---|
| (1S,1'S,2R,6'S,9'R,10'R)-3,3,10'-Trimethyl-2',6,11'-trioxo-3'-oxaspiro[cyclohexane-1,5'-tricyclo[7.2.1.0]dodecane]-4-ene-2-carbaldehyde |