MESNA
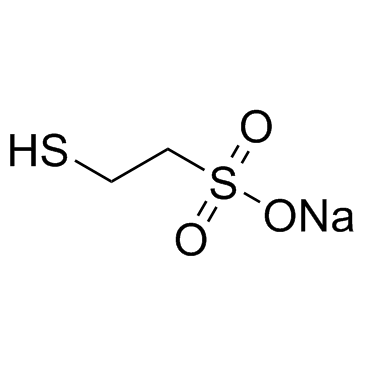
MESNA structure
|
Common Name | MESNA | ||
|---|---|---|---|---|
| CAS Number | 19767-45-4 | Molecular Weight | 164.179 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C2H5NaO3S2 | Melting Point | >240°C dec. | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of MESNA2-mercaptoethane sulfonate (Mesna), is a synthetic small molecule, widely used as a systemic protective agent against chemotherapy toxicity, but is primarily used to reduce hemorrhagic cystitis induced by cyclophosphamide.IC50 Value: 182 mM (decreased superoxide anion production stimulated with PMA (tetradecanoylphorbol acetate) in PMN in-vitro); 70mM (inhibited H2O2 production) [3]Target: in vitro: MESNA had no effect on the qualitative and quantitative characteristics of the indicated processes in both the types of the doxorubicin sensitive cells. The combined use of doxorubicin and phosphamide or cyclophosphane the use of MESNA for lowering the urotoxic action of oxazophosphorines had no effect on the biological efficacy of doxorubicin [4].in vivo: AMH-positive follicles were significantly decreased after cisplatin administration, which was significantly reversed when mesna was co-administered with cisplatin. The end product of lipid peroxidation, malondialdehyde (MDA), was significantly increased, but the anti-oxidative enzymatic activity of superoxide dismutase (SOD) and glutathione (GSH) were significantly decreased in cisplatin groups when compared with NS group. In contrast, after co-administration of cisplatin with mesna, MDA was significantly decreased whereas the activity of SOD and the concentration of GSH were increased. Moreover, mesna did not decrease the anti-tumor property of cisplatin in HePG2 cell lines [2]. After head trauma, tissue malondialdehyde levels increased; these levels were significantly decreased by MESNA administration. Caspase-3 levels were increased after trauma, but no effect of MESNA was determined in caspase-3 activity [1].Clinical trial: Effects of Mesna on Homocysteine in Kidney Failure . Phase2 |
| Name | Mesna |
|---|---|
| Synonym | More Synonyms |
| Description | 2-mercaptoethane sulfonate (Mesna), is a synthetic small molecule, widely used as a systemic protective agent against chemotherapy toxicity, but is primarily used to reduce hemorrhagic cystitis induced by cyclophosphamide.IC50 Value: 182 mM (decreased superoxide anion production stimulated with PMA (tetradecanoylphorbol acetate) in PMN in-vitro); 70mM (inhibited H2O2 production) [3]Target: in vitro: MESNA had no effect on the qualitative and quantitative characteristics of the indicated processes in both the types of the doxorubicin sensitive cells. The combined use of doxorubicin and phosphamide or cyclophosphane the use of MESNA for lowering the urotoxic action of oxazophosphorines had no effect on the biological efficacy of doxorubicin [4].in vivo: AMH-positive follicles were significantly decreased after cisplatin administration, which was significantly reversed when mesna was co-administered with cisplatin. The end product of lipid peroxidation, malondialdehyde (MDA), was significantly increased, but the anti-oxidative enzymatic activity of superoxide dismutase (SOD) and glutathione (GSH) were significantly decreased in cisplatin groups when compared with NS group. In contrast, after co-administration of cisplatin with mesna, MDA was significantly decreased whereas the activity of SOD and the concentration of GSH were increased. Moreover, mesna did not decrease the anti-tumor property of cisplatin in HePG2 cell lines [2]. After head trauma, tissue malondialdehyde levels increased; these levels were significantly decreased by MESNA administration. Caspase-3 levels were increased after trauma, but no effect of MESNA was determined in caspase-3 activity [1].Clinical trial: Effects of Mesna on Homocysteine in Kidney Failure . Phase2 |
|---|---|
| Related Catalog | |
| References |
| Melting Point | >240°C dec. |
|---|---|
| Molecular Formula | C2H5NaO3S2 |
| Molecular Weight | 164.179 |
| Exact Mass | 163.957779 |
| PSA | 104.38000 |
| LogP | 0.54220 |
| InChIKey | XOGTZOOQQBDUSI-UHFFFAOYSA-M |
| SMILES | O=S(=O)([O-])CCS.[Na+] |
| Storage condition | -20?C Freezer, Under Inert Atmosphere |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | KI7968000 |
| HS Code | 2930909090 |
|
~% 
MESNA CAS#:19767-45-4 |
| Literature: US2005/137419 A1, ; Page/Page column 4 ; |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Characterization and optimization of heroin hapten-BSA conjugates: method development for the synthesis of reproducible hapten-based vaccines.
Anal. Bioanal. Chem 406(24) , 5927-37, (2014) A potential new treatment for drug addiction is immunization with vaccines that induce antibodies that can abrogate the addictive effects of the drug of abuse. One of the challenges in the development... |
| sodium mercaptoethanesulfonate |
| 2-Mercaptoethanesulfonic acid monosodium salt |
| Mesnum |
| 2-Mercaptoethanesulfonic Acid Sodium Salt |
| Uromitexan |
| 2-mercaptoethanesulphonic acid sodium salt |
| 2-Mercaptoethanesulfonic acid sodium salt,Coenzyme M sodium salt,HS-CoM Na |
| Sodium 2-mercaptoethanesulfonate |
| sodium 2-Mercapto-ethanesulfonate |
| Ethanesulfonic acid, 2-mercapto-, sodium salt (1:1) |
| Sodium 2-sulfanylethanesulfonate |
| Ethanesulfonic acid, 2-mercapto-, monosodium salt |
| Mesnex |
| mesnaum |
| UNII-NR7O1405Q9 |
| mistabron |
| MFCD00007535 |
| MESNA |
| 2-Mercaptoethane sulfonate sodium |
| mucofluid |
| EINECS 243-285-9 |
| 2-Mercaptoethanesulfonate, sodium |
| mitexan |
| coenzyme M sodium salt |
 CAS#:45127-11-5
CAS#:45127-11-5 CAS#:35144-64-0
CAS#:35144-64-0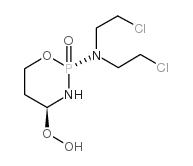 CAS#:39800-16-3
CAS#:39800-16-3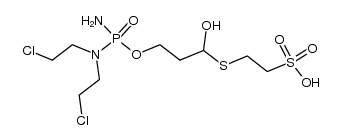 CAS#:105639-84-7
CAS#:105639-84-7 CAS#:84210-72-0
CAS#:84210-72-0
