Tranylcypromine hydrochloride

Tranylcypromine hydrochloride structure
|
Common Name | Tranylcypromine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1986-47-6 | Molecular Weight | 169.651 | |
| Density | N/A | Boiling Point | 218.3ºC at 760mmHg | |
| Molecular Formula | C9H12ClN | Melting Point | 162-169ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 90.8ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Tranylcypromine hydrochlorideTranylcypromine hydrochloride (SKF 385 hydrochloride) is an irreversible inhibitor of lysine-specific demethylase 1 (LSD1/BHC110) and monoamine oxidase (MAO). Tranylcypromine hydrochloride inhibits LSD1, MAO A and MAO B with IC50s of 20.7, 2.3 and 0.95 μM, respectively. Tranylcypromine hydrochloride can be used for the research of depression[1][2][3]. |
| Name | Tranylcypromine hydrochloride,(±)-trans-2-Phenylcyclopropylaminehydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Tranylcypromine hydrochloride (SKF 385 hydrochloride) is an irreversible inhibitor of lysine-specific demethylase 1 (LSD1/BHC110) and monoamine oxidase (MAO). Tranylcypromine hydrochloride inhibits LSD1, MAO A and MAO B with IC50s of 20.7, 2.3 and 0.95 μM, respectively. Tranylcypromine hydrochloride can be used for the research of depression[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
MAO-A:2.3 μM (IC50) MAO-B:0.95 μM (IC50) |
| In Vitro | Tranylcypromine hydrochlorid (50 μM-5 mM; 1 h or 12-14 h) inhibits histone and nucleosomal demethylation[1]. Tranylcypromine hydrochlorid (2 μM; 3 h) shows a specific derepression of OCT4 transcription[1]. Tranylcypromine hydrochlorid (0-100 μM; 15 min) shows IC50 values of 20.7, 2.3 and 0.95 μM for LSD1, MAO A and MAO B, respectively[2]. Tranylcypromine hydrochlorid (0-800 μM) shows Ki values of 242.7, 101.9 and 16 μM for LSD1, MAO A and MAO B, respectively[2]. Western Blot Analysis[1] Cell Line: Sf21 insect cell line Concentration: 50 μM, 200 μM, 1 mM and 5 mM Incubation Time: 12-14 hours or 1 hour Result: Showed inhibitory activities of histone H3K4 demethylation and nucleosomal demethylation. RT-PCR[1] Cell Line: P19 EC cell line Concentration: 2 μM Incubation Time: 3 hours Result: Decreased Oct4 mRNA levels. |
| In Vivo | Tranylcypromine hydrochlorid (3 mg/kg; i.p. once daily for 3 days) decreases LPS-mediated microglial activation and proinflammatory cytokine COX-2 and IL-6 levels in wild-type mice[3]. Tranylcypromine hydrochlorid (3 mg/kg; i.p. once daily for 7 days) down-regulates Aβ-mediate microglial activation in 5xFAD mice[3]. Animal Model: Wild-type mice[3] Dosage: 3 mg/kg Administration: Intraperitoneal injection; 3 mg/kg once daily for 3 days Result: Significantly down-regulated LPS-stimulated microglial activation in the cortex and Hippocampus, and LPS-induced astrocyte activation only in the cortex. Reduced LPS-induced COX-2 levels in hippocampus CA1, decreased LPS-evoked IL-6 levels in the cortex and hippocampus CA1 and suppressed LPS-mediated IL-1β levels in the cortex. Animal Model: 5xFAD mice[3] Dosage: 3 mg/kg Administration: Intraperitoneal injection; 3 mg/kg once daily for 7 days Result: Differentially regulated microglial and astrocyte activation in this mouse model of AD. |
| References |
| Boiling Point | 218.3ºC at 760mmHg |
|---|---|
| Melting Point | 162-169ºC(lit.) |
| Molecular Formula | C9H12ClN |
| Molecular Weight | 169.651 |
| Flash Point | 90.8ºC |
| Exact Mass | 169.065826 |
| PSA | 26.02000 |
| LogP | 3.00350 |
| Appearance of Characters | Powder or Chunks | White to light beige |
| Vapour Pressure | 0.127mmHg at 25°C |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 45-36/37/39-26 |
| RIDADR | UN 3249 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2921499090 |
|
~% 
Tranylcypromine... CAS#:1986-47-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 39, # 7 p. 1485 - 1493 |
|
~% 
Tranylcypromine... CAS#:1986-47-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 39, # 7 p. 1485 - 1493 |
|
~% 
Tranylcypromine... CAS#:1986-47-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 39, # 7 p. 1485 - 1493 |
| HS Code | 2921499090 |
|---|---|
| Summary | 2921499090 other aromatic monoamines and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals ... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predicti... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
| (1R,2S)-2-Phenylcyclopropanamine hydrochloride (1:1) |
| (1R,2S)-tranylcypromine hydrochloride |
| Cyclopropanamine, 2-phenyl-, (1R,2S)-, hydrochloride (1:1) |
| trans-2-Phenylcyclopropylamine hydrochloride |
| Tranylcypromine hydrochloride |
| trans-2-Phenylcyclopropanamine hydrochloride |
| MFCD00063602 |

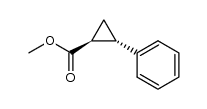
 CAS#:21087-29-6
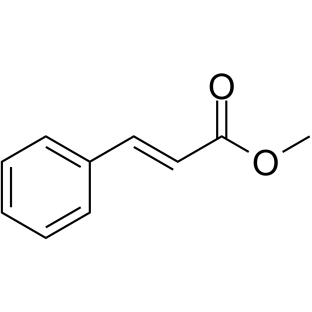
CAS#:21087-29-6 CAS#:4407-36-7
CAS#:4407-36-7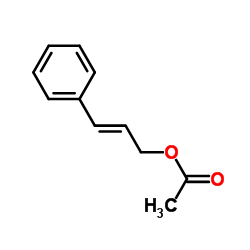 CAS#:21040-45-9
CAS#:21040-45-9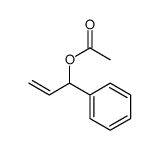 CAS#:7217-71-2
CAS#:7217-71-2
