pimozide
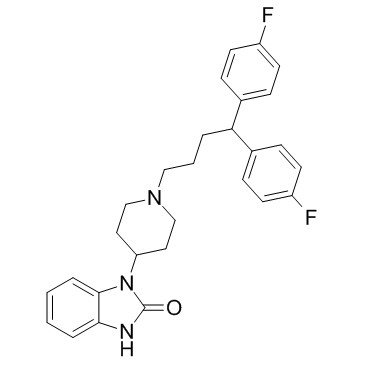
pimozide structure
|
Common Name | pimozide | ||
|---|---|---|---|---|
| CAS Number | 2062-78-4 | Molecular Weight | 461.546 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 649.0±65.0 °C at 760 mmHg | |
| Molecular Formula | C28H29F2N3O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 346.3±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of pimozidePimozide is a dopamine receptor antagonist, with Kis of 1.4 nM, 2.5 nM and 588 nM for dopamine D2, D3 and D1 receptors, respectively, and also has affinity at α1-adrenoceptor, with a Ki of 39 nM; Pimozide also inhibits STAT3 and STAT5. |
| Name | pimozide |
|---|---|
| Synonym | More Synonyms |
| Description | Pimozide is a dopamine receptor antagonist, with Kis of 1.4 nM, 2.5 nM and 588 nM for dopamine D2, D3 and D1 receptors, respectively, and also has affinity at α1-adrenoceptor, with a Ki of 39 nM; Pimozide also inhibits STAT3 and STAT5. |
|---|---|
| Related Catalog | |
| Target |
Dopamine D2 receptor:1.4 nM (Ki) Dopamine D3 receptor:2.5 nM (Ki) Dopamine D1 receptor:588 nM (Ki) α1-adrenoceptor:39 nM (Ki) STAT3 STAT5 |
| In Vitro | Pimozide is a dopamine receptor antagonist, with Kis of 1.4 nM, 2.5 nM and 588 nM for dopamine D2, D3 and D1 receptors, respectively; also has affinity at α1-adrenoceptor and 5-HT1A, with Kis of 39 nM and 310 nM, respectively[1]. Pimozide acts as an inhibitor of STAT3. Pimozide (0-15 μM) shows inhibitory of the proliferation of U2OS cells, with IC50 value at 24, 48, and 72 h of 22.16 ± 2.54, 17.49 ± 1.14 and 13.78 ± 0.34 μM, respectively. Pimozide (10 μM) inhibits the colony- and sphere-forming abilities of osteosarcoma cells. Pimozide (15 μM) induces G0/G1 phase cell cycle arrest, suppresses the extracellular signal-regulated kinase (Erk) signaling to inhibit cell viability, and produces ROS generation through inhibiting antioxidant enzyme gene catalase expression in osteosarcoma cells[2]. Pimozide acts as an inhibitor of STAT5. Pimozide reduces the expression of endogenous STAT5 target genes, and decreases STAT5 tyrosine phosphorylation[3]. |
| Cell Assay | Cell proliferation is assessed by WST-8 colorimetric assay. Human osteosarcoma cells are plated in 96-well plates with 2,500 cells per well and exposed to the treatment of different concentrations of pimozide for various time intervals (24 h, 48 h, and 72 h). The WST-8 solution is added to each well after indicated time. After incubated at 37°C for another 4 hours, the absorbance ismeasured at 450 nm using a multi-well plate reader[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 649.0±65.0 °C at 760 mmHg |
| Molecular Formula | C28H29F2N3O |
| Molecular Weight | 461.546 |
| Flash Point | 346.3±34.3 °C |
| Exact Mass | 461.227875 |
| PSA | 41.03000 |
| LogP | 6.06 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.623 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 18 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | DE1750000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
~98% 
pimozide CAS#:2062-78-4 |
| Literature: INSTITUT UNIVERSITARI DE CIENCIA I TECNOLOGIA Patent: WO2008/80601 A2, 2008 ; Location in patent: Page/Page column 10-11 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Simple and sensitive screening and quantitative determination of 88 psychoactive drugs and their metabolites in blood through LC–MS/MS: Application on postmortem samples
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 970 , 1-7, (2014) • The introduction has been modified. • More references have been added to the text. • Limitations of the method have been discussed more in detail. |
|
|
Factors influencing crystal growth rates from undercooled liquids of pharmaceutical compounds.
J. Phys. Chem. B 118(33) , 9974-82, (2014) Amorphous forms of drugs are increasingly being used to deliver poorly water-soluble compounds. Therefore, understanding the magnitude and origin of differences in crystallization kinetics is highly i... |
|
|
Progesterone downregulation of miR-141 contributes to expansion of stem-like breast cancer cells through maintenance of progesterone receptor and Stat5a.
Oncogene 34 , 3676-87, (2015) Progesterone (P4) has emerged as an important hormone-regulating mammary stem cell (MaSC) populations. In breast cancer, P4 and synthetic analogs increase the number of stem-like cells within luminal ... |
| pimozida |
| 1-[4,4-Di-(4-fluorophenyl)butyl]-4-(2-oxo-1-benzimidazolinyl)piperidine |
| Neoperidole |
| Pimozidum |
| 2H-Benzimidazol-2-one, 1-(1-(4,4-bis(4-fluorophenyl)butyl)-4-piperidinyl)-1,3-dihydro- |
| 1-{1-[4,4-Bis(4-fluorophenyl)butyl]-4-piperidinyl}-1,3-dihydro-2H-benzimidazol-2-one |
| pimozide |
| 1-[1-[4,4-bis(4-Fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazol-2-one |
| EINECS 218-171-7 |
| 3-[1-[4,4-bis(4-fluorophenyl)butyl]piperidin-4-yl]-1H-benzimidazol-2-one |
| 1H-Benzimidazol-2-ol, 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]- |
| 2H-Benzimidazol-2-one, 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro- |
| 1-[1-[4,4-bis(p-fluorophenyl)butyl]-4-piperidyl]-2-benzimidazolinone |
| Orap |
| McN-JR-6238 |
| 1-{1-[4,4-Bis(4-fluorophenyl)butyl]-4-piperidinyl}-1H-benzimidazol-2-ol |
| 1-{1-[4,4-Bis(4-fluorophenyl)butyl]piperidin-4-yl}-1,3-dihydro-2H-benzimidazol-2-one |
| Opiran |
| MFCD00055081 |
![1,1'-(4-chlorobutylidene)bis[4-fluorobenzene] structure](https://image.chemsrc.com/caspic/266/3312-04-7.png)