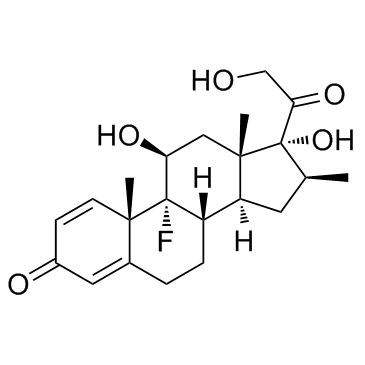
Betamethasone 17-valerate
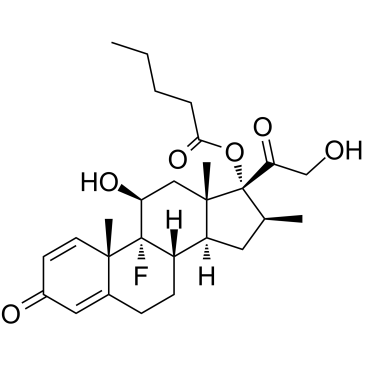
Betamethasone 17-valerate structure
|
Common Name | Betamethasone 17-valerate | ||
|---|---|---|---|---|
| CAS Number | 2152-44-5 | Molecular Weight | 476.578 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 598.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C27H37FO6 | Melting Point | 183-184ºC | |
| MSDS | Chinese USA | Flash Point | 316.0±30.1 °C | |
Use of Betamethasone 17-valerateBetamethasone valerate (Betamethasone 17-valerate), the 17-valerate ester of Betamethasone, is a topical corticosteroid with anti-inflammatory activity. Betamethasone valerate is used in the treatment of recurrent aphthous stomatitis. Betamethasone valerate inhibits the binding of the radiolabeled glucocorticoid dexamethasone (3H dexamethasone) to human epidermis and mouse skin with IC50s of 5 and 6 nM, respectively[1][2][3]. |
| Name | betamethasone valerate |
|---|---|
| Synonym | More Synonyms |
| Description | Betamethasone valerate (Betamethasone 17-valerate), the 17-valerate ester of Betamethasone, is a topical corticosteroid with anti-inflammatory activity. Betamethasone valerate is used in the treatment of recurrent aphthous stomatitis. Betamethasone valerate inhibits the binding of the radiolabeled glucocorticoid dexamethasone (3H dexamethasone) to human epidermis and mouse skin with IC50s of 5 and 6 nM, respectively[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vivo | Betamethasone valerate (10 mg; 5 minutes; applied to the irritated site with a micro spatula) ointment inhibits about 50% of the ear edema without thymus atrophy[4]. Betamethasone-17-valerate ointment (50 mg) inhibits homologous passive cutaneous anaphylaxis[4]. Animal Model: Female Sprague-Dawley rats weighing 60-70 g (croton oil edema experiment)[4] Dosage: 10 mg Administration: Applied to the irritated site with a micro spatula; 5 minutes Result: Inhibited about 50% of the ear edema without thymus atrophy. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 598.9±50.0 °C at 760 mmHg |
| Melting Point | 183-184ºC |
| Molecular Formula | C27H37FO6 |
| Molecular Weight | 476.578 |
| Flash Point | 316.0±30.1 °C |
| Exact Mass | 476.257416 |
| PSA | 100.90000 |
| LogP | 3.78 |
| Vapour Pressure | 0.0±3.8 mmHg at 25°C |
| Index of Refraction | 1.560 |
| Storage condition | 2-8°C |
|
Section 1. Chemical Product and Company Identification Betamethasone valerateCatalog BE185 Common Name/ Number(s). Trade Name CAS#2152-44-5
Manufacturer RTECSTU3835000 SPECTRUM QUALITY PRODUCTS INC. TSCATSCA 8(b) inventory: No products were found. Commercial Name(s)Not available. CI# Not available. Synonym9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione IN CASE OF EMERGENCY valerate Chemical NameNot available. Not available.CALL (310) 516-8000 Chemical Family Chemical FormulaC27H37FO6 SPECTRUM QUALITY PRODUCTS INC. Section 2.Composition and Information on Ingredients Exposure Limits TWA (mg/m3)STEL (mg/m3) CEIL (mg/m3) NameCAS #% by Weight 1) Betamethasone valerate2152-44-5100 Toxicological DataBetamethasone valerate: on IngredientsORAL (LD50):Acute: 4067 mg/kg [Rat]. Section 3. Hazards Identification Potential Acute Health Effects Very hazardous in case of ingestion. Hazardous in case of skin contact (sensitizer), of inhalation. Slightly hazardous in case of skin contact (irritant), of eye contact (irritant). Potential Chronic HealthVery hazardous in case of ingestion. EffectsHazardous in case of skin contact (sensitizer), of inhalation. Slightly hazardous in case of skin contact (irritant), of eye contact (irritant). CARCINOGENIC EFFECTS: Not available. MUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. The substance is toxic to lungs, mucous membranes. Repeated or prolonged exposure to the substance can produce target organs damage. Betamethasone valerate Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. WARM water MUST be used. Get medical attention if irritation occurs. Skin ContactIn case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Cover the irritated skin with an emollient. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. Serious Skin ContactWash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek medical attention. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention if symptoms appear. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2), halogenated compounds. Fire Hazards in Presence of Not available. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Special Remarks onNot available. Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Betamethasone valerate Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not breathe dust. Avoid contact with skin. Wear suitable protective clothing. In case of insufficient ventilation, wear suitable respiratory equipment. If ingested, seek medical advice immediately and show the container or the label. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Crystalline solid.)OdorOdorless. Not available. Taste Molecular Weight476.59 g/mole White. Color Not available. pH (1% soln/water) Boiling PointDecomposes. Melting Point183.5°C (362.3°F) Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. Not available. Volatility Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water, methanol, acetone. SolubilitySoluble in methanol, acetone. Very slightly soluble in cold water. Section 10. Stability and Reactivity Data StabilityThe product is stable. Instability TemperatureNot available. Conditions of InstabilityNot available. Incompatibility with various Not available. substances Betamethasone valerate CorrosivityNon-corrosive in presence of glass. Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsAcute oral toxicity (LD50): 4067 mg/kg [Rat]. Chronic Effects on Humans Causes damage to the following organs: lungs, mucous membranes. Other Toxic Effects onVery hazardous in case of ingestion. HumansHazardous in case of skin contact (sensitizer), of inhalation. Slightly hazardous in case of skin contact (irritant). Special Remarks onNot available. Toxicity to Animals Special Remarks onNot available. Chronic Effects on Humans Special Remarks on otherNot available. Toxic Effects on Humans Section 12. Ecological Information EcotoxicityNot available. Not available. BOD5 and COD Possibly hazardous short term degradation products are not likely. However, long term degradation products may Products of Biodegradation arise. Toxicity of the ProductsThe products of degradation are more toxic. of Biodegradation Not available. Special Remarks on the Products of Biodegradation Section 13. Disposal Considerations Waste Disposal Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). Not applicable. Identification Special Provisions forNot applicable. Transport Betamethasone valerate DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms No products were found. Federal and State Regulations California Proposition 65 Warnings Other RegulationsOSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). WHMIS (Canada) CLASS D-2A: Material causing other toxic effects (VERY TOXIC). Other Classifications DSCL (EEC)R43- May cause sensitization by skin contact. Health Hazard HMIS (U.S.A.)2 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 2 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Wear appropriate respirator when ventilation is inadequate. Betamethasone valerate SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R48 |
| Safety Phrases | S22-S24/25 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | TU3835000 |
| Hazard Class | 4.3 |
| HS Code | 2932999099 |
|
~% 
Betamethasone 1... CAS#:2152-44-5 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 37, # 12 p. 3286 - 3293 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Effects of topical corticosteroids on cell proliferation, cell cycle progression and apoptosis: in vitro comparison on HaCaT.
Int. J. Pharm. 479(2) , 422-9, (2015) Topical-corticosteroids are mainly used for the treatment of inflammatory or hyperproliferative skin diseases. The in vivo assay to rank topical-corticosteroids potency, based on the skin blanching, i... |
|
|
Therapeutic effects of minoxidil high extra combination therapy in patients with androgenetic alopecia.
Skinmed 10(5) , 276-82, (2012)
|
|
|
Genitogluteal porokeratosis: an unusual clinical presentation.
Australas. J. Dermatol. 53(2) , e36-9, (2012) A 51-year-old man presented with a 12-year history of an expanding, irritable rash on his buttocks, groin and scrotum. He gradually developed erythematous, annular plaques with ridged borders and depr... |
| (11β,16β)-9-fluoro-11,21-dihydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl pentanoate |
| Pregna-1,4-diene-3,20-dione, 9-fluoro-11,21-dihydroxy-16-methyl-17-((1-oxopentyl)oxy)-, (11β,16β)- |
| 9-Fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione-17-valerate |
| β-Methasone-17-valerate |
| (11b,16b)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 17-Valerate |
| (8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-17-(hydroxyacetyl)-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl pentanoate |
| Betamethasone valerate |
| VALISONE |
| Betamethasone 17-valerate |
| (11β,16β)-9-Fluoro-11,21-dihydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl valerate |
| 9-Fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17-valerate |
| UNII-9IFA5XM7R2 |
| MFCD00867446 |
| Pregna-1,4-diene-3,20-dione, 9-fluoro-11,21-dihydroxy-16-methyl-17-[(1-oxopentyl)oxy]-, (11β,16β)- |
| Hormezon |
| Ecoval 70 |
| Betnovate |
| Pentanoic acid, (11β,16β)-9-fluoro-11,21-dihydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl ester |
| β-Methasone 17-valerate |
| Celestoderm |
| EINECS 218-439-3 |