L-homoserine lactone hydrochloride
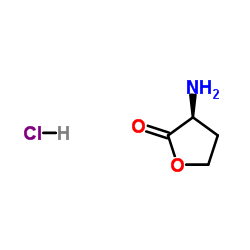
L-homoserine lactone hydrochloride structure
|
Common Name | L-homoserine lactone hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2185-03-7 | Molecular Weight | 137.56 | |
| Density | N/A | Boiling Point | 257.1ºC at 760 mmHg | |
| Molecular Formula | C4H8ClNO2 | Melting Point | 221-226ºC | |
| MSDS | Chinese USA | Flash Point | 120.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of L-homoserine lactone hydrochlorideL-Homoserine lactone hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | (3S)-3-aminooxolan-2-one,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | L-Homoserine lactone hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| References |
[1]. Koch, T., and Buchardt, O.Synthesis of L-(+)-selenomethionineSynthesis1065-1067(1993) |
| Boiling Point | 257.1ºC at 760 mmHg |
|---|---|
| Melting Point | 221-226ºC |
| Molecular Formula | C4H8ClNO2 |
| Molecular Weight | 137.56 |
| Flash Point | 120.1ºC |
| Exact Mass | 137.024353 |
| PSA | 52.32000 |
| LogP | 0.76290 |
| Vapour Pressure | 0.000411mmHg at 25°C |
| Index of Refraction | -26 ° (C=0.2, H2O) |
| InChIKey | XBKCXPRYTLOQKS-DFWYDOINSA-N |
| SMILES | Cl.NC1CCOC1=O |
| Storage condition | 2~8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932209090 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Intergenerational cultural conflict, mental health, and educational outcomes among Asian and Latino/a Americans: Qualitative and meta-analytic review.
Psychol. Bull. 141(2) , 404-46, (2015) Among immigrant Asian and Latino groups, the contrast between collectivism in traditional heritage and individualism in the mainstream American cultures presents unique challenges for their family rel... |
|
|
Catalytic asymmetric construction of spiro(γ-butyrolactam-γ-butyrolactone) moieties through sequential reactions of cyclic imino esters with Morita-Baylis-Hillman bromides.
Chemistry 18(40) , 12614-8, (2012) Spiro(γ-butyrolactam-γ-butyrolactone): a route to enantioenriched spiro(γ-butyrolactam-γ-butyrolactone) compounds, a valuable motif for drug discovery, was developed by use of a highly efficient coppe... |
|
|
[The influence of bacterial autoregulation molecules (homoserine lactones and alkyloxybenzoles) on oxidative metabolism of the natural immunity cellular effectors].
Mikrobiologiia 82(2) , 147-56, (2013)
|
| (S)-α-Amino-γ-butyrolactone hydrochloride |
| (S)-(-)-α-Amino-γ-butyrolactone Hydrochloride |
| 2(3H)-Furanone, 3-aminodihydro-, (3S)-, hydrochloride (1:1) |
| (S)-3-Aminodihydrofuran-2(3H)-one hydrochloride |
| (S)-(-)-3-Aminotetrahydrofuran-2-one Hydrochloride |
| (3S)-3-Aminodihydrofuran-2(3H)-one hydrochloride (1:1) |
| (S)-(+)-|A-amino-|A-butyrolactone hydrochloride |
| L-(-)-Homoserine Lactone Hydrochloride |
| EINECS 218-571-1 |
| L-Homoserine lactone hydrochloride |
| L-Homoserine lactone HCl |
| (2S)-homoserine lactone hydrochloride |
| LSHLO,HCl |
| (S)-Homoserine-lactone hydrochloride |
| MFCD00058172 |
| (3S)-3-Aminodihydro-2(3H)-furanone hydrochloride (1:1) |
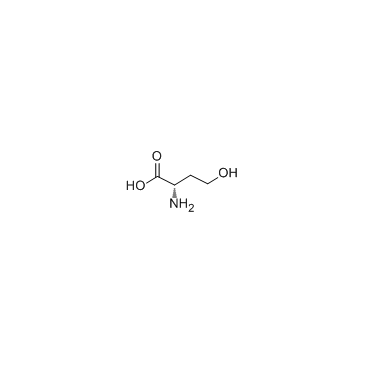 CAS#:672-15-1
CAS#:672-15-1 CAS#:33693-66-2
CAS#:33693-66-2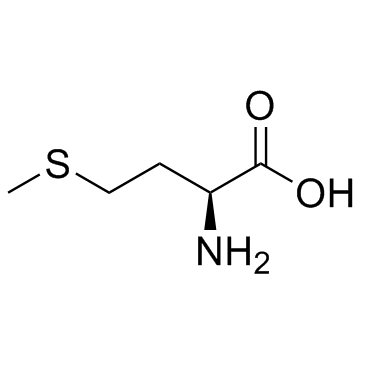 CAS#:63-68-3
CAS#:63-68-3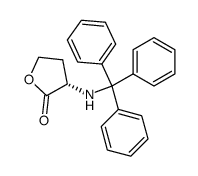 CAS#:83427-81-0
CAS#:83427-81-0 CAS#:498-19-1
CAS#:498-19-1![(3S)-4-methoxy-4-oxo-3-[(2,2,2-trifluoroacetyl)amino]butanoate Structure](https://image.chemsrc.com/caspic/146/657-19-2.png) CAS#:657-19-2
CAS#:657-19-2 CAS#:104347-03-7
CAS#:104347-03-7 CAS#:56-84-8
CAS#:56-84-8 CAS#:42417-39-0
CAS#:42417-39-0 CAS#:40856-59-5
CAS#:40856-59-5 CAS#:35677-89-5
CAS#:35677-89-5 CAS#:10405-07-9
CAS#:10405-07-9 CAS#:177158-19-9
CAS#:177158-19-9 CAS#:15159-65-6
CAS#:15159-65-6 CAS#:168982-69-2
CAS#:168982-69-2
