Imatinib Mesylate (STI571)
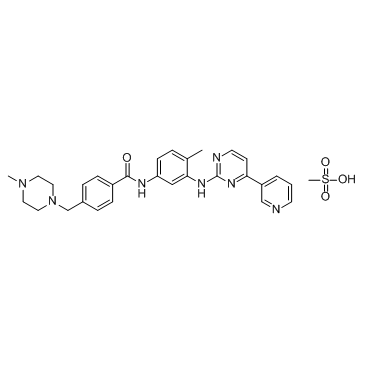
Imatinib Mesylate (STI571) structure
|
Common Name | Imatinib Mesylate (STI571) | ||
|---|---|---|---|---|
| CAS Number | 220127-57-1 | Molecular Weight | 589.708 | |
| Density | 0.858 g/mL at 25 °C(lit.) | Boiling Point | 133-134 °C(lit.) | |
| Molecular Formula | C30H35N7O4S | Melting Point | 214-224°C | |
| MSDS | Chinese USA | Flash Point | 64°F | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Imatinib Mesylate (STI571)Imatinib Mesylate is a tyrosine kinases inhibitor that inhibits c-Kit, Bcr-Abl, and PDGFR (IC50=100 nM) tyrosine kinases. |
| Name | imatinib methanesulfonate |
|---|---|
| Synonym | More Synonyms |
| Description | Imatinib Mesylate is a tyrosine kinases inhibitor that inhibits c-Kit, Bcr-Abl, and PDGFR (IC50=100 nM) tyrosine kinases. |
|---|---|
| Related Catalog | |
| Target |
IC50: ~100 nM (c-Kit, Bcr-Abl, and PDGFR)[1] |
| In Vitro | Imatinib (STI571) Mesylate inhibits c-Kit autophosphorylation, activation of MAPK, and activation of Akt without altering total protein levels of c-kit, MAPK, or Akt. The concentration that produces 50% inhibition for these effects is approximately 100 nM[1]. Imatinib (STI571) mesylate is very effective (in vitro IC50 of 25 nM) against the chronic myeloid leukemia-causing kinase Bcr-Abl. Imatinib also efficiently inhibits Kit (in vitro IC50, 410 nM) and PDGFR (in vitro IC50, 380 nM)[2]. Imatinib (STI571) mesylate is a multi-target inhibitor of v-Abl, c-Kit and inhibits Bcr/Abl, v-Abl, Tel/Abl, the native PDGFβ receptor, and c-Kit, but it does not inhibit Src family kinases, c-Fms, Flt3, the EGFR or multiple other tyrosine kinases. Imatinib inhibits tyrosine phosphorylation and cell growth of Ba/F3 cells expressing Bcr/Abl, Tel/Abl, Tel/PDGFβR, and Tel/Arg with an IC50 of approximately 0.5 μM in each case, but it has no effect on untransformed Ba/F3 cells growing in IL-3 or on Ba/F3 cells transformed by Tel/JAK2[3]. Imatinib mesylate selectively inhibits the activity of Bcr/Abl, c-Kit and PDGFR kinases. Imatinib mesylate reveals distinct and rapid antileukemic activity in chronic myelogenous leukemia (CML) and Philadelphia-positive (Ph+) acute lymphoblastic leukemia (ALL)[4]. |
| In Vivo | Animals treated with Imatinib Mesylate show a decrease of mean body weight throughout the whole study. Body weight loss is noticeable in mice from groups that receive chemotherapy and the vitamin D analog combined treatment. The body weight decrease of mice treat with both combined Imatinib mesylate and PRI-2191 is the highest (15%) on Day 22 of the experiment, but after that day, mice start to recover[4]. In a rat Ischemia/reperfusion injury (IRI) model, Imatinib mesylate attenuates lung injury by an antipermeability and antiinflammatory effect. The delivery and function of Imatinib mesylate in the lung is also confirmed in this model[5]. |
| Cell Assay | Tested A549 cells are placed in 96-well flat-bottom plates at a density of 5×103 cells per well 24 h before the addition of the test compounds. The cells are incubated for 96 h with two different concentrations (10 and 100 nM) of PRI-2191 and concurrently with various concentrations of Imatinib mesylate (10, 100, 1000 and 10,000 ng/mL) and other cytostatic drugs (Docetaxel (DTX) or Idarubicin (ID) : 0.1, 1, 10, 100 ng/mL; Cisplatin (CIS): 1, 10, 100, 1000 ng/mL). The sulforhodamine B (SRB) assay is performed to evaluate the cytotoxic effect. As a result, IC50 is calculated for each separate experiment in Cheburator 0.4, Dmitry Nevozhay software[4]. |
| Animal Admin | Mice[4] NOD/SCID female mice, 12-16 weeks old, body weight of 20-25 g, are used. Mice are subcutaneously (s.c.) inoculated in the right flank of the abdomen with A549 tumor cells suspension (5×106 cells in 0.2 mL of Hank’s medium per mouse, Day 0) and then are randomized into groups receiving varied combinations of vitamin D analogs and chemotherapeutics. One out of two experimental protocols is applied in the respective experiments: 1. The treatment is started from Day 7 after inoculation of tumor cells (when tumors become palpable). Imatinib mesylate is administered intraperitoneally (i.p.) at a dose of 75 mg/kg/day, daily for 19 days (from Days 7-25). PRI-2191 is administered s.c. or by oral gavage at a dose of 2 μg/kg/day, 3 times a week (on Days 7, 12, 14, 16, 19, 21 and 23). 2. The treatment is started from Day 7 after inoculation of tumor cells (when tumors become palpable). Imatinib mesylate is administered intraperitoneally (i.p.) at a dose of 50 mg/kg/day, daily for 13 days (from Days 7-19). PRI-2191 and PRI-2205 are administered s.c. at doses of 1 or 10 μg/kg/day, respectively, 3 times a week (on Days 7, 10, 12, 14, 17, 19, 21, 24 and 26). At the end of the experiments, blood is collected under anesthesia; then, the mice are sacrificed. Rats[5] Male Lewis rats weighing 270 to 320 g are used in the experiments. Imatinib mesylate (50 mg/kg) is injected intraperitoneally in the Imatinib group (n=7), and 0.5 mL of 20% DMSO without Imatinib is administered in the vehicle group (n=7). The dose of 25 mg/kg is preliminarily tested, and it produces a little improvement in lung function without statistical significance. The dose of 50 mg/kg and intraperitoneal administration are adopted based on this result and past reports. The animals undergo left thoracotomy, and the left hilum is occluded with a small metallic clamp. The occlusion is performed 20 minutes after Imatinib or vehicle administration. During clamping, the tidal volume (TV) and respiratory rate (RR) are adjusted to 8 mL/kg and 80 breaths/min, respectively. After 90 minutes of ischemia, the clamp is removed and reperfusion is maintained for 120 minutes. During reperfusion, blood flow and ventilation are restored in the bilateral lung. In the sham group (n=6), the animals are heparinized, thoracotomized, and ventilated for 210 minutes. |
| References |
[3]. Okuda K, et al. ARG tyrosine kinase activity is inhibited by STI571.Blood. 2001 Apr 15;97(8):2440-8 |
| Density | 0.858 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 133-134 °C(lit.) |
| Melting Point | 214-224°C |
| Molecular Formula | C30H35N7O4S |
| Molecular Weight | 589.708 |
| Flash Point | 64°F |
| Exact Mass | 589.247131 |
| PSA | 149.03000 |
| LogP | 5.19690 |
| Index of Refraction | n20/D 1.401(lit.) |
| InChIKey | YLMAHDNUQAMNNX-UHFFFAOYSA-N |
| SMILES | CS(=O)(=O)O.Cc1ccc(NC(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Nc1nccc(-c2cccnc2)n1 |
| Storage condition | -20°C Freezer |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| Safety Phrases | S16-S26-S36/37/39 |
| RIDADR | UN 1993 3/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 3 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Paradoxical and contradictory effects of imatinib in two cell line models of hormone-refractory prostate cancer.
Prostate 75 , 923-35, (2015) Imatinib mesylate is a chemotherapeutic drug that inhibits the tyrosine kinase activity of c-KIT and has been successfully used to treat leukemias and some solid tumors. However, its application for t... |
|
|
Evaluation of the toxic effects of four anti-cancer drugs in plant bioassays and its potency for screening in the context of waste water reuse for irrigation.
Chemosphere 135 , 403-10, (2015) Anti-cancer drugs are compounds that are of high environmental relevance because of their lack of specific mode of action. They can be extremely harmful to living organisms even at low concentrations.... |
|
|
Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in spinal cord and forebrain.
Glia 64 , 122-38, (2016) Transcription factors of the SoxD protein family have previously been shown to prevent precocious specification and terminal differentiation of oligodendrocyte progenitor cells in the developing spina... |
| 4-[(4-Methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide methanesulfonate |
| MFCD04307699 |
| Imatinib mesylate |
| Benzamide, 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-, methanesulfonate (1:1) |
| Imatinib mesilate (JAN) |
| Methansulfonsäure--4-[(4-methylpiperazin-1-yl)methyl]-N-{4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl}benzolcarboxamid(1:1) |
| 4-[(4-Methyl-1-piperazinyl)methyl]-N-(4-methyl-3-{[4-(3-pyridinyl)-2-pyrimidinyl]amino}phenyl)benzamide methanesulfonate |
| Imantinib mesylate |
| 4-[(4-Methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide methanesulfonate (1:1) |
| Gleevac |
| GLEEVEC |
| Imatinib mesilate |
| 4-[(4-Methyl-1-piperazinyl)methyl]-N-(4-methyl-3-{[4-(3-pyridinyl)-2-pyrimidinyl]amino}phenyl)benzamide methanesulfonate (1:1) |
| Glivec |
| STI-571 |

