6-Hydroxymelatonin
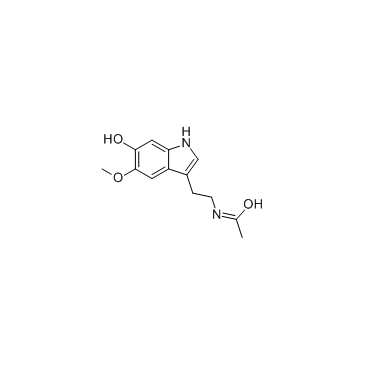
6-Hydroxymelatonin structure
|
Common Name | 6-Hydroxymelatonin | ||
|---|---|---|---|---|
| CAS Number | 2208-41-5 | Molecular Weight | 248.27800 | |
| Density | 1.266g/cm3 | Boiling Point | 564.7ºC at 760mmHg | |
| Molecular Formula | C13H16N2O3 | Melting Point | 172-175ºC | |
| MSDS | Chinese USA | Flash Point | 295.3ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of 6-Hydroxymelatonin6-Hydroxymelatonin is a primary metabolic of Melatonin, which is metabolized by cytochrome P450 (CYP) 1A2. |
| Name | 6-hydroxymelatonin |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Hydroxymelatonin is a primary metabolic of Melatonin, which is metabolized by cytochrome P450 (CYP) 1A2. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.266g/cm3 |
|---|---|
| Boiling Point | 564.7ºC at 760mmHg |
| Melting Point | 172-175ºC |
| Molecular Formula | C13H16N2O3 |
| Molecular Weight | 248.27800 |
| Flash Point | 295.3ºC |
| Exact Mass | 248.11600 |
| PSA | 74.35000 |
| LogP | 1.95160 |
| Vapour Pressure | 2.35E-13mmHg at 25°C |
| Index of Refraction | 1.627 |
| InChIKey | OMYMRCXOJJZYKE-UHFFFAOYSA-N |
| SMILES | COc1cc2c(CCNC(C)=O)c[nH]c2cc1O |
| Storage condition | Refrigerator |
| Water Solubility | alcohol: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | 22-40 |
| Safety Phrases | 36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AC3607000 |
| HS Code | 2933990090 |
|
~64% 
6-Hydroxymelatonin CAS#:2208-41-5 |
| Literature: Karam, Omar; Zunino, Fabien; Chagnaut, Vincent; Jouannetaud, Marie Paule; Jacquesy, Jean Claude Tetrahedron Letters, 2003 , vol. 44, # 7 p. 1511 - 1513 |
|
~% 
6-Hydroxymelatonin CAS#:2208-41-5 |
| Literature: Tetrahedron Letters, , vol. 44, # 7 p. 1511 - 1513 |
|
~% 
6-Hydroxymelatonin CAS#:2208-41-5 |
| Literature: Tetrahedron Letters, , vol. 44, # 7 p. 1511 - 1513 |
|
~% 
6-Hydroxymelatonin CAS#:2208-41-5 |
| Literature: Tetrahedron Letters, , vol. 44, # 7 p. 1511 - 1513 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro.
Mol. Cell. Endocrinol. 404 , 1-8, (2015) Melatonin and its metabolites including 6-hydroxymelatonin (6(OH)M), N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (AFMK) and 5-methoxytryptamine (5MT) are endogenously produced in human epidermis. This... |
|
|
Predominance of 2-hydroxymelatonin over melatonin in plants.
J. Pineal Res. 59 , 448-54, (2015) The cloning of the gene encoding melatonin 2-hydroxylase (M2H), which is responsible for the synthesis of 2-hydroxymelatonin, has expanded the study of melatonin metabolism in plants. Kinetic analysis... |
|
|
Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa).
J. Pineal Res. 58(3) , 343-51, (2015) Although melatonin biosynthetic genes from plants have been cloned, the melatonin catabolism mechanisms remain unclear. To clone the genes responsible for melatonin metabolism, we ectopically expresse... |
| 6-HydroxyMelatonin |
| 6-Hydroxy Melatonin |
| N-[2-(6-hydroxy-5-methoxy-1H-indol-3-yl)ethyl]acetamide |
![3-[2-(Acetylamino)ethyl]-5-Methoxy-6-acetyloxy-1H-indole-1-carboxylic Acid Ethyl Ester structure](https://image.chemsrc.com/caspic/319/519186-55-1.png)
![3-[2-(Acetylamino)ethyl]-5-Methoxy-1H-indole-1-carboxylic Acid Ethyl Ester structure](https://image.chemsrc.com/caspic/357/519186-54-0.png)
![6-Acetyl-3-[2-(acetylamino)ethyl]-5-Methoxy-H-indole-1-carboxylic Acid Ethyl Ester structure](https://image.chemsrc.com/caspic/350/188397-05-9.png)