Alsterpaullone
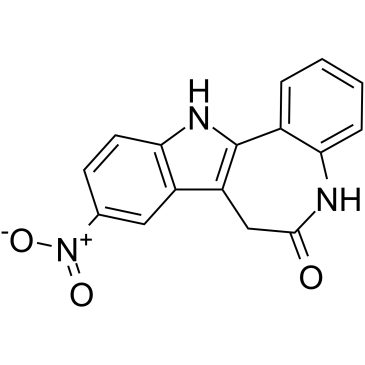
Alsterpaullone structure
|
Common Name | Alsterpaullone | ||
|---|---|---|---|---|
| CAS Number | 237430-03-4 | Molecular Weight | 293.277 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 651.5±45.0 °C at 760 mmHg | |
| Molecular Formula | C16H11N3O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 347.8±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AlsterpaulloneAlsterpaullone (9-Nitropaullone;NSC 705701) is a potent cyclin-dependent kinases (CDK) inhibitor, with IC50s of 35 nM, 15 nM, 200 nM and 40 nM for CDK1/cyclin B, CDK2/cyclin A, CDK2/cyclin E and CDK5/p35, respectively. Alsterpaullone also competes with ATP for binding to glycogen synthase kinase-3 alpha/beta (GSK-3alpha/GSK-3beta) with an IC50 of 4 nM, with antitumor activity and potential for the treatment of neurodegenerative and proliferative disorders[1]. |
| Name | Alsterpaullone |
|---|---|
| Synonym | More Synonyms |
| Description | Alsterpaullone (9-Nitropaullone;NSC 705701) is a potent cyclin-dependent kinases (CDK) inhibitor, with IC50s of 35 nM, 15 nM, 200 nM and 40 nM for CDK1/cyclin B, CDK2/cyclin A, CDK2/cyclin E and CDK5/p35, respectively. Alsterpaullone also competes with ATP for binding to glycogen synthase kinase-3 alpha/beta (GSK-3alpha/GSK-3beta) with an IC50 of 4 nM, with antitumor activity and potential for the treatment of neurodegenerative and proliferative disorders[1]. |
|---|---|
| Related Catalog | |
| Target |
Cdk1/cyclin B:35 nM (IC50) cdk2/cyclin A:15 nM (IC50) CDK2/Cyc E:200 nM (IC50) CDK5/p35:40 nM (IC50) GSK-3α:4 (IC50) GSK-3β:4 nM (IC50) |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 651.5±45.0 °C at 760 mmHg |
| Molecular Formula | C16H11N3O3 |
| Molecular Weight | 293.277 |
| Flash Point | 347.8±28.7 °C |
| Exact Mass | 293.080048 |
| PSA | 90.71000 |
| LogP | 3.55 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.738 |
| InChIKey | OLUKILHGKRVDCT-UHFFFAOYSA-N |
| SMILES | O=C1Cc2c([nH]c3ccc([N+](=O)[O-])cc23)-c2ccccc2N1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~32% 
Alsterpaullone CAS#:237430-03-4 |
| Literature: Zaharevitz, Daniel W.; Gussio, Rick P.; Jalluri, Ravi K.; Sausville, Edward A.; Kunick, Conrad; Meijer, Laurent Patent: US2002/42412 A1, 2002 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Paullones, a series of cyclin-dependent kinase inhibitors: synthesis, evaluation of CDK1/cyclin B inhibition, and in vitro antitumor activity.
J. Med. Chem. 42 , 2909, (1999) The paullones represent a novel class of small molecule cyclin-dependent kinase (CDK) inhibitors. To investigate structure-activity relationships and to develop paullones with antitumor activity, deri... |
|
|
Evidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute mania.
Int. J. Neuropsychopharmacol. 14 , 1051-67, (2011) There is a growing body of evidence suggesting that animal models can be developed to probe the specific domains of bipolar disorder (BD) using the endophenotype approach. Here we tested clinically ac... |
|
|
Induction of canonical Wnt signaling by alsterpaullone is sufficient for oral tissue fate during regeneration and embryogenesis in Nematostella vectensis.
Dev. Dyn. 240 , 2673-9, (2011) Although regeneration is widespread among metazoa, the molecular mechanisms have been studied in only a handful of taxa. Of these taxa, fewer still are amenable to studies of embryogenesis. Our unders... |
| 9-Nitro-7,12-dihydroindolo[3,2-d][1]benzazepin-6(5H)-one |
| 9-Nitro-7,12-dihydroindolo-[3,2-d][1]benzazepin-6(5)-one |
| Indolo[3,2-d][1]benzazepin-6(5H)-one, 7,12-dihydro-9-nitro- |
| 9-nitro-7,12-dihydro-5H-indolo[3,2-d][1]benzazepin-6-one |