Catharanthine
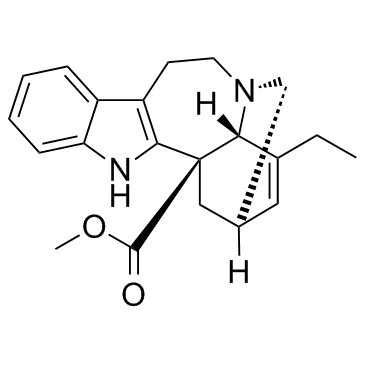
Catharanthine structure
|
Common Name | Catharanthine | ||
|---|---|---|---|---|
| CAS Number | 2468-21-5 | Molecular Weight | 336.427 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 491.5±45.0 °C at 760 mmHg | |
| Molecular Formula | C21H24N2O2 | Melting Point | 138-140ºC | |
| MSDS | USA | Flash Point | 251.1±28.7 °C | |
Use of CatharanthineCatharanthine inhibits nicotinic receptor mediated diaphragm contractions with IC50 of 59.6 μM.Target: nAChRCatharanthine evokes a concentration-dependent attenuation of carbachol responses in the rat ileum preparation, producing rightward curve displacements and decreases in maximal agonist responses. The mixture of serpentine, plus ajmalicine and catharanthine reveals a concentration-dependent inhibitory effect of acethylcholinesterase (AchE), with an IC50 at ca. 2.25 μg/Ml [1]. Catharanthine can induce the self-association of tubulin into linear indefinite polymers with an efficacy that is 75% that of vinblastine or vincristine. Catharanthine binds to tubulin alpha-beta dimer with binding constant of 2.8 mM [2]. Catharanthine stimulates release of amylase from pancreatic fragments and to cause extensive degranulation of pancreatic acinar cells with accumulation of membrane material in the Golgi region. Catharanthine induces a delayed release of Ca2+ from prelabeled pancreatic fragments as compared to bethanechol [3]. Catharanthine inhibits epibatidine-induced Ca(2+) influx in TE671-α, -β, -γ, -δ cells in a noncompetitive manner with similar potencies IC50 of 17 mM-25 mM. Catharanthine inhibits [3H]TCP binding to the desensitized Torpedo AChR with higher affinity compared to the resting AChR. Catharanthine enhances [3H]cytisine binding to resting but activatable Torpedo AChRs, suggesting desensitizing properties [4]. |
| Name | catharanthine |
|---|---|
| Synonym | More Synonyms |
| Description | Catharanthine inhibits nicotinic receptor mediated diaphragm contractions with IC50 of 59.6 μM.Target: nAChRCatharanthine evokes a concentration-dependent attenuation of carbachol responses in the rat ileum preparation, producing rightward curve displacements and decreases in maximal agonist responses. The mixture of serpentine, plus ajmalicine and catharanthine reveals a concentration-dependent inhibitory effect of acethylcholinesterase (AchE), with an IC50 at ca. 2.25 μg/Ml [1]. Catharanthine can induce the self-association of tubulin into linear indefinite polymers with an efficacy that is 75% that of vinblastine or vincristine. Catharanthine binds to tubulin alpha-beta dimer with binding constant of 2.8 mM [2]. Catharanthine stimulates release of amylase from pancreatic fragments and to cause extensive degranulation of pancreatic acinar cells with accumulation of membrane material in the Golgi region. Catharanthine induces a delayed release of Ca2+ from prelabeled pancreatic fragments as compared to bethanechol [3]. Catharanthine inhibits epibatidine-induced Ca(2+) influx in TE671-α, -β, -γ, -δ cells in a noncompetitive manner with similar potencies IC50 of 17 mM-25 mM. Catharanthine inhibits [3H]TCP binding to the desensitized Torpedo AChR with higher affinity compared to the resting AChR. Catharanthine enhances [3H]cytisine binding to resting but activatable Torpedo AChRs, suggesting desensitizing properties [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 491.5±45.0 °C at 760 mmHg |
| Melting Point | 138-140ºC |
| Molecular Formula | C21H24N2O2 |
| Molecular Weight | 336.427 |
| Flash Point | 251.1±28.7 °C |
| Exact Mass | 336.183777 |
| PSA | 45.33000 |
| LogP | 4.05 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.663 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~96% 
Catharanthine CAS#:2468-21-5 |
| Literature: Szantay, Csaba; Boelcskei, Hedvig; Gacs-Baitz, Eszter Tetrahedron, 1990 , vol. 46, # 5 p. 1711 - 1732 |
|
~% 
Catharanthine CAS#:2468-21-5 |
| Literature: Tetrahedron, , vol. 46, # 5 p. 1711 - 1732 |
|
~% 
Catharanthine CAS#:2468-21-5 |
| Literature: Tetrahedron, , vol. 46, # 5 p. 1711 - 1732 |
|
~% 
Catharanthine CAS#:2468-21-5 |
| Literature: Tetrahedron, , vol. 46, # 5 p. 1711 - 1732 |
|
Catharanthine dilates small mesenteric arteries and decreases heart rate and cardiac contractility by inhibition of voltage-operated calcium channels on vascular smooth muscle cells and cardiomyocytes.
J. Pharmacol. Exp. Ther. 345(3) , 383-92, (2013) Catharanthine is a constituent of anticancer vinca alkaloids. Its cardiovascular effects have not been investigated. This study compares the in vivo hemodynamic as well as in vitro effects of catharan... |
|
|
The leaf epidermome of Catharanthus roseus reveals its biochemical specialization.
Plant Cell 20(3) , 524-42, (2008) Catharanthus roseus is the sole commercial source of the monoterpenoid indole alkaloids (MIAs), vindoline and catharanthine, components of the commercially important anticancer dimers, vinblastine and... |
|
|
Simultaneous determination of vinblastine and its monomeric precursors vindoline and catharanthine in Catharanthus roseus by capillary electrophoresis-mass spectrometry.
J. Sep. Sci. 34 , 2885-2892, (2011) Catharanthus roseus is an important dicotyledonous medicinal plant that contains various anticancer components, such as vinblastine (VLB) and its monomeric precursors (vindoline and catharanthine). A ... |
| Ibogamine-18-carboxylic acid, 3,4-didehydro-, methyl ester, (2α,5β,18β)- |
| Methyl (2α,5β,18β)-3,4-didehydroibogamine-18-carboxylate |
| Catharanthine Sulphate |
| catharintine |
| CatharanthineSulfateBase |
| catharantine |
| (2α,5β,6α,18β)-3,4-Didehydroibogamine-18-carboxylic acid methyl ester |
| Ibogamine-18-carboxylic acid, 3,4-didehydro-, methyl ester, (2a,5b,6a,18b)- |
| (+)-3,4-Didehydrocoronaridine |
| Catharanthine |
| catharanthin |
| UNII-WT0YJV846J |
| catharinthine |
| MFCD01753356 |
| Ibogamine-18-carboxylic acid, 3,4-didehydro-, methyl ester, (2α,5β,6α,18β)- |

![(-)-2-(1-[2-(indol-3-yl)-1-oxo-ethyl])-6-ethyl-7-exo-chloro-2-azabicyclo[2.2.2]oct-5-ene-7-endo-carboxylic acid methyl ester structure](https://image.chemsrc.com/caspic/403/129030-48-4.png)
 CAS#:38390-45-3
CAS#:38390-45-3 CAS#:23360-92-1
CAS#:23360-92-1 CAS#:63944-54-7
CAS#:63944-54-7
