Cefazolin

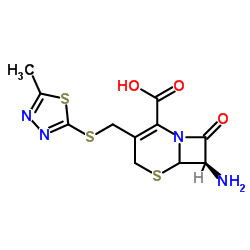
Cefazolin structure
|
Common Name | Cefazolin | ||
|---|---|---|---|---|
| CAS Number | 25953-19-9 | Molecular Weight | 454.507 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C14H14N8O4S3 | Melting Point | 198-200ºC | |
| MSDS | N/A | Flash Point | N/A | |
Use of CefazolinCefazolin is an antibiotic used for the research of a number of anti-bacterial infections. Cefazolin can be used for the prophylaxis of surgical antimicrobial. Cefazolin has anti-inflammatory effect and can attenuate post-operative cognitive dysfunction (POCD)[1]. |
| Name | cefazolin |
|---|---|
| Synonym | More Synonyms |
| Description | Cefazolin is an antibiotic used for the research of a number of anti-bacterial infections. Cefazolin can be used for the prophylaxis of surgical antimicrobial. Cefazolin has anti-inflammatory effect and can attenuate post-operative cognitive dysfunction (POCD)[1]. |
|---|---|
| Related Catalog | |
| Target |
Antibiotic[1] |
| In Vitro | Cefazolin (50-300 μg/mL; 6 or 24 hours) has a direct anti-inflammatory effect on C8-B4 cells stimulated by LPS (1 μg/mL)[1]. Cell Viability Assay[1] Cell Line: Mouse C8-B4 microglial cells Concentration: 50, 100, 150, 200, 250, and 300 μg/mL Incubation Time: 6 or 24 hours Result: Inhibited proinflammatory cytokine production. Inhibited IL-6 production at 50 and 100 μg/mL. |
| In Vivo | Cefazolin (300 mg/kg; injected intraperitoneally 1 h before surgery and then once per day for 5 days after surgery) can attenuate surgery-induced post-operative memory and learning impairment in mice. Cefazolin alone may induce cognitive dysfunction possibly by transient gut dysbiosis in mice without surgery[1]. Animal Model: Six- to 8-week-old CD-1 male mice (weighing 31-36 g) [1] Dosage: 300-500 mg/kg Administration: 10 mg in 0.1 mL was intraperitoneally injected 30 min before surgery and then once every day for 5 days. Result: Improved learning and memory after surgery and might impair learning and memory in mice without surgery. |
| References |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Melting Point | 198-200ºC |
| Molecular Formula | C14H14N8O4S3 |
| Molecular Weight | 454.507 |
| Exact Mass | 454.029999 |
| PSA | 234.93000 |
| LogP | 1.13 |
| Index of Refraction | 1.961 |
| InChIKey | MLYYVTUWGNIJIB-BXKDBHETSA-N |
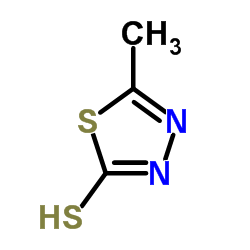
| SMILES | Cc1nnc(SCC2=C(C(=O)O)N3C(=O)C(NC(=O)Cn4cnnn4)C3SC2)s1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~76% 
Cefazolin CAS#:25953-19-9 |
| Literature: WO2005/20904 A2, ; Page/Page column 19 ; |
|
~92% 
Cefazolin CAS#:25953-19-9 |
| Literature: Tetrahedron, , vol. 41, # 22 p. 5133 - 5139 |
|
~70% 
Cefazolin CAS#:25953-19-9 |
| Literature: Tetrahedron Letters, , vol. 38, # 26 p. 4693 - 4696 |
|
~78% 
Cefazolin CAS#:25953-19-9 |
| Literature: Journal of Organic Chemistry, , vol. 62, # 26 p. 9099 - 9106 |
|
~% 
Cefazolin CAS#:25953-19-9 |
| Literature: Tetrahedron Letters, , vol. 38, # 26 p. 4693 - 4696 |
|
~% 
Cefazolin CAS#:25953-19-9 |
| Literature: Tetrahedron Letters, , vol. 38, # 26 p. 4693 - 4696 |
|
~% 
Cefazolin CAS#:25953-19-9 |
| Literature: Journal of Organic Chemistry, , vol. 62, # 26 p. 9099 - 9106 |
|
~% 
Cefazolin CAS#:25953-19-9 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 44, # 3 p. 599 - 601 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 3004909090 |
|---|
|
Isolation of Escherichia coli strains with AcrAB-TolC efflux pump-associated intermediate interpretation or resistance to fluoroquinolone, chloramphenicol and aminopenicillin from dogs admitted to a university veterinary hospital.
J. Vet. Med. Sci. 76(7) , 937-45, (2014) Understanding the prevalence of antimicrobial-resistance and the relationship between emergence of resistant bacteria and clinical treatment can facilitate design of effective treatment strategies. We... |
|
|
Aspiration sclerotherapy combined with pasireotide to improve reduction of large symptomatic hepatic cysts (SCLEROCYST): study protocol for a randomized controlled trial.
Trials 16 , 82, (2015) Aspiration sclerotherapy is an effective therapeutic option for large symptomatic hepatic cysts. However, incomplete cyst reduction following aspiration sclerotherapy is frequently reported. Strong po... |
|
|
Skeletal muscle and plasma concentrations of cefazolin during cardiac surgery in infants.
J. Thorac. Cardiovasc. Surg. 148(6) , 2634-41, (2014) To describe the pharmacokinetics and tissue disposition of prophylactic cefazolin into skeletal muscle in a pediatric population undergoing cardiac surgery.The subjects included 12 children, with a me... |
| Cephazolin |
| 7-(1-(1H)-Tetrazolylacetamido)-3-[2-(5-methyl-1,3,4-thiadiazolyl)thiomethyl]-D3-cephem-4-carboxylic Acid |
| Cephamezine |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[2-(1H-tetrazol-1-yl)acetyl]amino]-, (6R,7R)- |
| Cefazolinum |
| Cefazolina |
| Cephazoline |
| 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[(1H-tetrazol-1-ylacetyl)amino]-, (6R,7R)- |
| (6R,7R)-3-[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanylmethyl]-8-oxo-7-[[2-(tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cefazoline |
| (6R,7R)-3-{[(5-Methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl}-8-oxo-7-[(1H-tetrazol-1-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cephazolidin |
| Ancef |
| MFCD00243010 |
| EINECS 247-362-8 |
| Cefazolin |
| (6R)-3-(5-methyl-[1,3,4]thiadiazol-2-ylsulfanylmethyl)-8-oxo-7t-(2-tetrazol-1-yl-acetylamino)-(6rH)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| (6R,7R)-3-{[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl}-8-oxo-7-[(1H-tetrazol-1-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cefamezin |
| Cefazolin Acid |

![(6R-trans)-7-amino-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrochloride structure](https://image.chemsrc.com/caspic/246/98493-37-9.png)
![7-((tetrazol-1'-yl)acetylamino)-3-acetyloxymethyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid structure](https://image.chemsrc.com/caspic/499/32510-61-5.png)