naproxen sodium
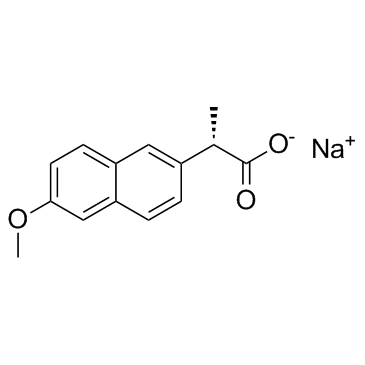
naproxen sodium structure
|
Common Name | naproxen sodium | ||
|---|---|---|---|---|
| CAS Number | 26159-34-2 | Molecular Weight | 252.241 | |
| Density | N/A | Boiling Point | 403.9ºC at 760 mmHg | |
| Molecular Formula | C14H13NaO3 | Melting Point | 250-251ºC | |
| MSDS | Chinese USA | Flash Point | 154.5ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of naproxen sodiumNaproxen sodium is a COX-1 and COX-2 inhibitor with IC50s of 8.72 and 5.15 μM, respectively in cell assay. |
| Name | naproxen sodium |
|---|---|
| Synonym | More Synonyms |
| Description | Naproxen sodium is a COX-1 and COX-2 inhibitor with IC50s of 8.72 and 5.15 μM, respectively in cell assay. |
|---|---|
| Related Catalog | |
| Target |
COX-2:5.65 μM (IC50, in intact cells ) COX-1:9.55 μM (IC50, in intact cells ) |
| In Vitro | Naproxen etemesil is a lipophilic, non-acidic, inactive prodrug of naproxen that is hydrolysed to pharmacologically active Naproxen once absorbed. Naproxen is a well known nonsteroidal anti-inflammatory drug. Naproxen is approximately equipotent inhibitor of COX-1 and COX-2 in intact cells with IC50s of 2.2 μg/mL and 1.3 μg/mL, respectively[1]. |
| In Vivo | Naproxen exerts an anti-inflammatory and antifibrotic effect in mouse model of bleomycin-induced lung fibrosis. Naproxen also downregulates TGF-β levels and Smad3/4 complex formation[2]. Naproxen is shown to inhibit the time-courses of pain, fever and PGE2 with similar potencies (IC50=27, 40, 13 μM)[3]. |
| Cell Assay | BAEC are incubated for 30 min with Naproxen (0.1 ng/mL to 1 mg/mL). Arachidonic acid (30 μM) is then added, and the cells are incubated for a further 15 min at 37°C. The medium is then removed, and radioimmunoassay is used to measure the formation of 6-keto-PGF,a, PGE2, thromboxane B2, or PGF2a[1]. |
| Animal Admin | Rats[3] To measure the analgesic effects of naproxen in a carrageenaninduced model of monoarthritis, Male Sprague–Dawley rats (n=48, 217±28 g) are randomly divided into four groups of 12 by an internally developed computer program, allowing the blind performance of the behavioral experiment. To induce hyperalgesia by inflammation, animals in groups 1B, 1C, and 1D receive a 40-μL intra-articular injection of a saline solution containing 7.5 mg/mL carrageenan in the left hind limb under isoflurane anesthesia (time=−1 h). Animals in group 1A receive no injection. After 1 h (time=0) the animals in groups 1A, 1B, 1C, and 1D receive oral doses of naproxen in saline of 0, 0, 7.5 and 30 μmol/kg, respectively. The doses and time points of measurements are selected on the basis of simulations predicting measuring a full concentration-effect relationship within the time-span of the experiment[3]. Mice[2] Bleomycin (0.05 IU) is instilled intratracheally to C57BL/6 mice, which are then treated by micro-osmotic pump with vehicle, JNJ7777120 (40 mg/kg b.wt.), naproxen (21 mg/kg b.wt.), or a combination of both. Airway resistance to inflation, an index of lung stiffness, is assessed, and lung specimens are processed for inflammation, oxidative stress, and fibrosis markers[2]. |
| References |
| Boiling Point | 403.9ºC at 760 mmHg |
|---|---|
| Melting Point | 250-251ºC |
| Molecular Formula | C14H13NaO3 |
| Molecular Weight | 252.241 |
| Flash Point | 154.5ºC |
| Exact Mass | 252.076233 |
| PSA | 49.36000 |
| LogP | 1.70180 |
| Vapour Pressure | 3.01E-07mmHg at 25°C |
| InChIKey | CDBRNDSHEYLDJV-FVGYRXGTSA-M |
| SMILES | COc1ccc2cc(C(C)C(=O)[O-])ccc2c1.[Na+] |
| Storage condition | Desiccate at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H360 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| Safety Phrases | 53-45 |
| RIDADR | UN 3249 |
| RTECS | QJ1047000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2918990090 |
|
~% 
naproxen sodium CAS#:26159-34-2 |
| Literature: DR. REDDY'S LABORATORIES LTD.; DR. REDDY'S LABORATORIES, INC. Patent: WO2009/149053 A2, 2009 ; Location in patent: Page/Page column 28 ; |
|
~% 
naproxen sodium CAS#:26159-34-2 |
| Literature: Albemarle Corporation Patent: US5936118 A1, 1999 ; |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Ion-exchange molecularly imprinted polymer for the extraction of negatively charged acesulfame from wastewater samples.
J. Chromatogr. A. 1411 , 23-33, (2015) Acesulfame is a known indicator that is used to identify the introduction of domestic wastewater into water systems. It is negatively charged and highly water-soluble at environmental pH values. In th... |
|
|
HPLC method for naproxen determination in human plasma and its application to a pharmacokinetic study in Turkey.
J. Chromatogr. Sci. 52(7) , 584-9, (2014) A simple high-performance liquid chromatography method has been developed for the determination of naproxen in human plasma. The method was validated on an Ace C18 column using ultraviolet detection. ... |
|
|
A new analytical method to determine non-steroidal anti-inflammatory drugs in surface water using in situ derivatization combined with ultrasound-assisted emulsification microextraction followed by gas chromatography-mass spectrometry.
Talanta 129 , 552-9, (2014) Because of the high stability and potential toxic effects of non-steroidal anti-inflammatory drugs (NSAIDs), it is important to closely monitor their concentrations in the environment using a sensitiv... |
| EINECS 247-486-2 |
| sodium (S)-2-(6-methoxynaphthalen-2-yl)propanoate |
| Anaprox,Naprelan |
| (S)-Naproxen Sodium Salt |
| Naproxen sodium |
| MFCD00058507 |
| Naproxen (sodium) |
 CAS#:163133-43-5
CAS#:163133-43-5