7-Azaindole
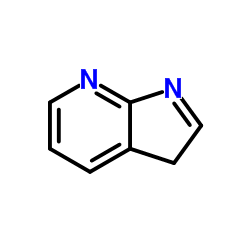
7-Azaindole structure
|
Common Name | 7-Azaindole | ||
|---|---|---|---|---|
| CAS Number | 271-63-6 | Molecular Weight | 118.14 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 283.1±40.0 °C at 760 mmHg | |
| Molecular Formula | C7H6N2 | Melting Point | 105-107 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 125.0±27.3 °C | |
Use of 7-Azaindole1H-Pyrrolo[2,3-b]pyridine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 7-Azaindole |
|---|---|
| Synonym | More Synonyms |
| Description | 1H-Pyrrolo[2,3-b]pyridine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 283.1±40.0 °C at 760 mmHg |
| Melting Point | 105-107 °C(lit.) |
| Molecular Formula | C7H6N2 |
| Molecular Weight | 118.14 |
| Flash Point | 125.0±27.3 °C |
| Exact Mass | 118.053101 |
| PSA | 28.68000 |
| LogP | -0.64 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.657 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36 |
| Safety Phrases | S39-S26-S36/37/39 |
| RIDADR | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| RTECS | UY8710000 |
| HS Code | 2933990090 |

7-Azaindole CAS#:271-63-6 ~% 
7-Azaindole CAS#:271-63-6 |
| Literature: Park, Sun-Young; Jeong, Hyeok; Jang, Du-Jeon Journal of Physical Chemistry B, 2011 , vol. 115, # 19 p. 6023 - 6031 |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Selectivity of kinase inhibitor fragments.
J. Med. Chem. 54 , 5131-43, (2011) A kinase-focused screening set of fragments has been assembled and has proved successful for the discovery of ligand-efficient hits against many targets. Here we present some of our general conclusion... |
|
|
Synthesis of 7-azaserotonin: its photophysical properties associated with excited state proton transfer reaction.
J. Am. Chem. Soc. 128 , 14426, (2006) We report the synthesis of 3-(2-aminoethyl)-5-ol-1H-pyrrolo[2,3-b]pyridine (7-azaserotonin), which may potentially serve as an agonist or antagonist of serotonin receptors. In alcohols, the solvent (e... |
|
|
A practical synthesis of 2-((1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino)-5- fluoronicotinic acid.
J. Org. Chem. 71 , 4021-4023, (2006) A practical synthesis of a key pharmaceutical intermediate, 2-[(1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino]-5-fluoronicotinic acid (1), is described. To introduce the aminomethyl moiety of 2 via a pal... |
| 1H-Pyrrolo[2,3-b]pyridine |
| 7-Aza-7H-indole |
| 3H-Pyrrolo[2,3-b]pyridine |
| 7-Dideazapurine |
| MFCD00005606 |
| 1H-Pyrrolo(2,3-b)pyridine |
| EINECS 205-981-0 |
| 7-Aza-1-pyrindine |
| 1,7-Diazaindene |
![2-{1H-pyrrolo[2,3-b]pyridin-1-yl}acetic acid structure](https://image.chemsrc.com/caspic/398/1048913-13-8.png) CAS#:1048913-13-8
CAS#:1048913-13-8![1,3-Dihydro-2H-pyrrolo[2,3-b]pyridin-2-on structure](https://image.chemsrc.com/caspic/077/5654-97-7.png) CAS#:5654-97-7
CAS#:5654-97-7 CAS#:357263-13-9
CAS#:357263-13-9 CAS#:55052-27-2
CAS#:55052-27-2![methyl 2-oxo-2-(1H-pyrrolo[2,3-b]pyridin-3-yl)acetate structure](https://image.chemsrc.com/caspic/424/357263-49-1.png) CAS#:357263-49-1
CAS#:357263-49-1![1H-Pyrrolo[2,3-b]pyridin-2-carbaldehyd structure](https://image.chemsrc.com/caspic/139/394223-03-1.png) CAS#:394223-03-1
CAS#:394223-03-1 CAS#:319474-34-5
CAS#:319474-34-5 CAS#:55052-24-9
CAS#:55052-24-9 CAS#:5654-92-2
CAS#:5654-92-2![1H-Pyrrolo[2,3-b]pyridine-2,3-dione structure](https://image.chemsrc.com/caspic/153/5654-95-5.png) CAS#:5654-95-5
CAS#:5654-95-5
