Mesaconitine
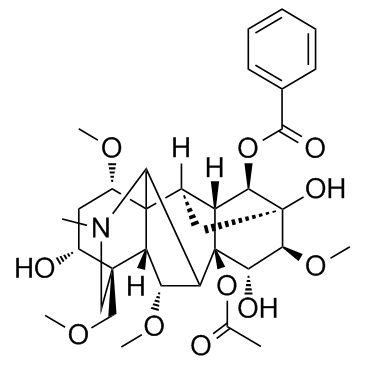
Mesaconitine structure
|
Common Name | Mesaconitine | ||
|---|---|---|---|---|
| CAS Number | 2752-64-9 | Molecular Weight | 631.711 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 695.0±55.0 °C at 760 mmHg | |
| Molecular Formula | C33H45NO11 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 374.1±31.5 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of MesaconitineMesaconitine is the main active component of genus aconitum plants.IC50 value:Target: in vitro: In HUVECs, 30 microM mesaconitine increased the [Ca(2+)](i) level in the presence of extracellular CaCl(2) and NaCl, and the response was inhibited by KBR7943. Mesaconitine increased intracellular Na(+) concentration level in HUVECs. The [Ca(2+)](i) response by mesaconitine was inhibited by 100 microM D-tubocurarine [1]. Mesaconitine at 30 microM inhibited 3 microM phenylephrine-induced contraction in the endothelium-intact, but not endothelium-denuded, aortic rings [2]. MA promoted the alpha-MT-induced decrease in NE levels in hippocampus, medulla oblongata plus pons and spinal cord [3]. |
| Name | Mesaconitine |
|---|---|
| Synonym | More Synonyms |
| Description | Mesaconitine is the main active component of genus aconitum plants.IC50 value:Target: in vitro: In HUVECs, 30 microM mesaconitine increased the [Ca(2+)](i) level in the presence of extracellular CaCl(2) and NaCl, and the response was inhibited by KBR7943. Mesaconitine increased intracellular Na(+) concentration level in HUVECs. The [Ca(2+)](i) response by mesaconitine was inhibited by 100 microM D-tubocurarine [1]. Mesaconitine at 30 microM inhibited 3 microM phenylephrine-induced contraction in the endothelium-intact, but not endothelium-denuded, aortic rings [2]. MA promoted the alpha-MT-induced decrease in NE levels in hippocampus, medulla oblongata plus pons and spinal cord [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 695.0±55.0 °C at 760 mmHg |
| Molecular Formula | C33H45NO11 |
| Molecular Weight | 631.711 |
| Flash Point | 374.1±31.5 °C |
| Exact Mass | 631.299255 |
| PSA | 153.45000 |
| LogP | 0.27 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.618 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 + H330 |
| Precautionary Statements | P260-P264-P284-P301 + P310-P310 |
| Hazard Codes | Xn |
| Safety Phrases | 24/25 |
| RIDADR | UN 1544 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
|
~88% 
Mesaconitine CAS#:2752-64-9 |
| Literature: Kulanthaivel, Palaniappan; Pelletier, S. William Tetrahedron, 1988 , vol. 44, # 14 p. 4313 - 4320 |
|
~% 
Mesaconitine CAS#:2752-64-9 |
| Literature: Kulanthaivel, Palaniappan; Pelletier, S. William Tetrahedron, 1988 , vol. 44, # 14 p. 4313 - 4320 |
| Precursor 3 | |
|---|---|
| DownStream 1 | |
|
Vasorelaxing effect of mesaconitine, an alkaloid from Aconitum japonicum, on rat small gastric artery: possible involvement of endothelium-derived hyperpolarizing factor Mitamura M, et al
Jpn. J. Pharmacol. 89(4) , 380-7, (2002)
|
| 13,14,15-pentol,1,6,16-trimethoxy-4-(methoxymethyl)-20-methyl-aconitane-8 |
| Mesaaconitine |
| Aconitane-3,8,13,14,16-pentol, 1,6,15-trimethoxy-4-(methoxymethyl)-20-methyl-, 8-acetate 14-benzoate |
| N-Desethyl-N-Methylaconitine |
| MESACONITINE(SH) |
| Japaconitine B |
| Japaconitine A |
| 8-Acetoxy-3,13,16-trihydroxy-1,6,15-trimethoxy-4-(methoxymethyl)-20-methylaconitan-14-yl benzoate |
| MESACONITINE (PRIMARY STANDARD) |

 CAS#:6900-87-4
CAS#:6900-87-4
