Gimatecan
Modify Date: 2025-08-26 14:11:56
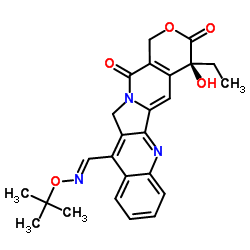
Gimatecan structure
|
Common Name | Gimatecan | ||
|---|---|---|---|---|
| CAS Number | 292618-32-7 | Molecular Weight | 447.483 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 780.6±70.0 °C at 760 mmHg | |
| Molecular Formula | C25H25N3O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 425.9±35.7 °C | |
Use of GimatecanGimatecan (ST1481) is a potent topoisomerase I inhibitor. Gimatecan is an orally bioavailable camptothecin analogue with antitumor activity[1]. |
| Name | 7-[(E)-tert-butyloxyiminomethyl]-camptothecin |
|---|---|
| Synonym | More Synonyms |
| Description | Gimatecan (ST1481) is a potent topoisomerase I inhibitor. Gimatecan is an orally bioavailable camptothecin analogue with antitumor activity[1]. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I |
| In Vitro | Gimatecan (3 to 300 ng/mL) significantly inhibits the growth of human bladder cancer models (HT1376 and MCR), thus reflecting antiproliferative potency[1]. Gimatecan causes a persistent S-phase arrest At 0.003 µg/mL and the number of S-phase cells increased after treatment with a higher concentration (0.03 µg/mL)[1]. Cell Proliferation Assay[1] Cell Line: HT1376 cells harbor a p53 mutation; MCR cells harbor two p53 mutations: one in exon 4 (CGC→CCC) and one in exon 9 (CAG→TAG) Concentration: 3 to 300 ng/mL Incubation Time: 1, 6, and 24 hours Result: IC50s of 90±3 and 9.0±0.4 ng/mL for MCR and HT1376 cells after treatment for 1 hours. IC50s of 5.0±0.2 and 2.8±0.1 ng/mL for MCR and HT1376 cells after treatment for 24 hours. The growth-inhibitory effect was dose-dependent and time-dependent. HT1376 cells were more sensitive than MCR cells at least following a short-term exposure. |
| In Vivo | Gimatecan (2 mg/kg; treatment per os, every fourth day for four times) is effective for inhibiting tumor growth[1]. Animal Model: Athymic Swiss nude mice bearing HT1376 model[1] Dosage: 2 mg/kg Administration: Treatment per os, every fourth day for four times Result: Caused a marked tumor growth inhibition during treatment. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 780.6±70.0 °C at 760 mmHg |
| Molecular Formula | C25H25N3O5 |
| Molecular Weight | 447.483 |
| Flash Point | 425.9±35.7 °C |
| Exact Mass | 447.179413 |
| PSA | 103.01000 |
| LogP | 3.42 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.667 |
| InChIKey | UIVFUQKYVFCEKJ-OPTOVBNMSA-N |
| SMILES | CCC1(O)C(=O)OCc2c1cc1n(c2=O)Cc2c-1nc1ccccc1c2C=NOC(C)(C)C |
| Hazard Codes | T+ |
|---|
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| trans-1-Hydroxy-7-phenyl-hepten-(2)-diin-(4,6) |
| 7-Phenyl-hept-2t-en-4,6-diin-1-ol |
| 7-tert-butoxyiminomethylcamptothecin |
| (E)-7-phenyl-2-hepten-4,6-diyn-1-ol |
| 7-phenyl-hept-2t-ene-4,6-diyn-1-ol |
| ST-1481 |
| 1-Phenyl-hepten-(5)-trans-diin-(1.3)-ol-(7) |
| trans-7-Phenyl-hepten-(2)-diin-(4.6)-ol-(1) |
| 2-Heptene-4,6-diyn-1-ol,7-phenyl-,(E) |
| (4S)-11-[(E)-(tert-butoxyimino)methyl]-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| 7-(t-butoxy)iminomethylcamptothecin |
| 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-11-carboxaldehyde, 4-ethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-, 11-(O-(1,1-dimethylethyl)oxime), (4S)- |
| (E)-7-tert-butoxyiminomethyl-camptothecin |
| 7-hydroxy-1-phenyl-hept-5E-ene-1,3-diyne |
| (4S)-4-Ethyl-4-hydroxy-11-[(E)-{[(2-methyl-2-propanyl)oxy]imino}methyl]-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-11-carboxaldehyde, 4-ethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-, 11-[O-(1,1-dimethylethyl)oxime], (4S)- |
| Gimatecan |
 CAS#:39684-28-1
CAS#:39684-28-1![(S)-11-DimethoxyMethyl-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione Structure](https://image.chemsrc.com/caspic/376/84017-99-2.png) CAS#:84017-99-2
CAS#:84017-99-2