(R)-CR8
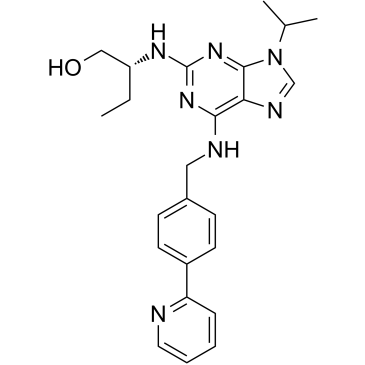
(R)-CR8 structure
|
Common Name | (R)-CR8 | ||
|---|---|---|---|---|
| CAS Number | 294646-77-8 | Molecular Weight | 431.533 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 671.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C24H29N7O | Melting Point | N/A | |
| MSDS | USA | Flash Point | 359.8±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (R)-CR8(R)-CR8 (CR8), a second-generation analog of Roscovitine, is a potent CDK1/2/5/7/9 inhibitor. (R)-CR8 (CR8) inhibits CDK1/cyclin B (IC50=0.09 μM), CDK2/cyclin A (0.072 μM), CDK2/cyclin E (0.041 μM), CDK5/p25 (0.11 μM), CDK7/cyclin H (1.1 μM), CDK9/cyclin T (0.18 μM) and CK1δ/ε (0.4 μM). (R)-CR8 (CR8) induces apoptosis and has neuroprotective effect[1][2]. |
| Name | (2R)-2-[[9-propan-2-yl-6-[(4-pyridin-2-ylphenyl)methylamino]purin-2-yl]amino]butan-1-ol |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-CR8 (CR8), a second-generation analog of Roscovitine, is a potent CDK1/2/5/7/9 inhibitor. (R)-CR8 (CR8) inhibits CDK1/cyclin B (IC50=0.09 μM), CDK2/cyclin A (0.072 μM), CDK2/cyclin E (0.041 μM), CDK5/p25 (0.11 μM), CDK7/cyclin H (1.1 μM), CDK9/cyclin T (0.18 μM) and CK1δ/ε (0.4 μM). (R)-CR8 (CR8) induces apoptosis and has neuroprotective effect[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Cdk1/cyclin B:0.09 μM (IC50) cdk2/cyclin A:0.072 μM (IC50) CDK2/cyclinE:0.041 μM (IC50) Cdk5/p25:0.11 μM (IC50) CDK7/cyclin H:1.1 μM (IC50) CDK9/Cyclin T:0.18 μM (IC50) CK1δ/ε:0.4 μM (IC50) |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 671.4±65.0 °C at 760 mmHg |
| Molecular Formula | C24H29N7O |
| Molecular Weight | 431.533 |
| Flash Point | 359.8±34.3 °C |
| Exact Mass | 431.243347 |
| PSA | 100.78000 |
| LogP | 2.10 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Natural product extracts of the Canadian prairie plant, Thermopsis rhombifolia, have anti-cancer activity in phenotypic cell-based assays.
Nat. Prod. Res. 29 , 1026-34, (2015) Many plant species within the terrestrial ecological zones of Canada have not yet been investigated for anti-cancer activity. We examined the scientific literature describing the endemic flora from th... |
|
|
Substitutions in the Escherichia coli RNA polymerase inhibitor T7 Gp2 that allow inhibition of transcription when the primary interaction interface between Gp2 and RNA polymerase becomes compromised.
Microbiology 158 , 2753-64, (2012) The Escherichia coli-infecting bacteriophage T7 encodes a 7 kDa protein, called Gp2, which is a potent inhibitor of the host RNA polymerase (RNAp). Gp2 is essential for T7 phage development. The inter... |
|
|
Delayed expression of cell cycle proteins contributes to astroglial scar formation and chronic inflammation after rat spinal cord contusion.
J. Neuroinflammation 9 , 169, (2012) Traumatic spinal cord injury (SCI) induces secondary tissue damage that is associated with astrogliosis and inflammation. We previously reported that acute upregulation of a cluster of cell-cycle-rela... |
| (2R)-2-[(9-Isopropyl-6-{[4-(2-pyridinyl)benzyl]amino}-9H-purin-2-yl)amino]-1-butanol |
| 3ddp |
| CR-8 |
| HMS3229B13 |
| 1-Butanol, 2-[[9-(1-methylethyl)-6-[[[4-(2-pyridinyl)phenyl]methyl]amino]-9H-purin-2-yl]amino]-, (2R)- |
![2-chloro-9-isopropyl-N-[[4-(2-pyridyl)phenyl]methyl]purin-6-amine structure](https://www.chemsrc.com/caspic/148/294648-01-4.png)