Narciclasine
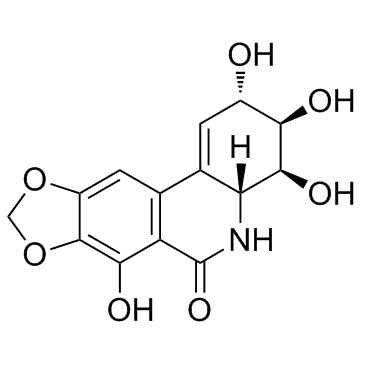
Narciclasine structure
|
Common Name | Narciclasine | ||
|---|---|---|---|---|
| CAS Number | 29477-83-6 | Molecular Weight | 307.255 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 733.8±60.0 °C at 760 mmHg | |
| Molecular Formula | C14H13NO7 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 397.6±32.9 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of NarciclasineNarciclasine is a plant growth modulator. Narciclasine modulates the Rho/Rho kinase/LIM kinase/cofilin signaling pathway, greatly increasing GTPase RhoA activity as well as inducing actin stress fiber formation in a RhoA-dependent manner. |
| Name | Narciclasine |
|---|---|
| Synonym | More Synonyms |
| Description | Narciclasine is a plant growth modulator. Narciclasine modulates the Rho/Rho kinase/LIM kinase/cofilin signaling pathway, greatly increasing GTPase RhoA activity as well as inducing actin stress fiber formation in a RhoA-dependent manner. |
|---|---|
| Related Catalog | |
| Target |
ROCK |
| In Vitro | Narciclasine activates Rho and stress fibers in glioblastoma multiforme cells. The mean IC50 of ~50 nM calculated on the 6 human glioblastoma multiforme (U373, Hs683, GL19, GL5, GL16, GL17), The mean IC50 value of 47 nM for Narciclasine across a panel of 60 cancer cell lines[1]. Bioassay-guided fractionation of the A. belladonna extract resulted in the identification of lycorine as the bio-active compound. The IC50 measured for radicle growth inhibition is 0.1 µM for Narciclasine[2]. |
| In Vivo | The i.v. regimen of Narciclasine at 1 mg/kg significantly (P=0.02) increases the survival of GL19 glioblastoma multiforme-bearing mice. Narciclasine when given orally at the same dose five times a week for 5 consecutive weeks also significantly increases animal survival in this model (P=0.008). Oral treatment with Narciclasine at 1 mg/kg significantly increases the survival (P=0.004) of Hs683 glioblastoma multiforme-bearing mice. Increasing the number of doses administered per week does not increase the survival of these Hs683 glioblastoma multiforme-bearing mice. Narciclasine appears to show similar increased survival in these models to temozolomide but at appreciable lower doses and following both oral and i.v. administration[1]. |
| Kinase Assay | The RhoA G-LISA assay is used. The assay uses a 96-well plate coated with the Rho binding domain of Rho family effector proteins. The active GTP-bound form of the Rho family protein but not the inactive GDP-bound form from biological samples will bind to the plate. Bound active Rho family protein is then detected by incubation with a specific primary antibody followed by a secondary antibody conjugated to horseradish peroxidase. The signal is then developed using OPD. U373 and GL19 glioblastoma multiforme cells are serum starved for 24 h and left untreated or treated with Narciclasine (100 nM) for 3 min, 5 min, 30 min, 1 h, and 2 h or treated with 2.0 μg/mL of the cell-permeable Rho inhibitor for 4 h in serum-free medium at 37°C with and without subsequent Narciclasine treatment. This product consists of highly purified C3 transferase covalently linked to a proprietary cell-penetrating moiety via a disulfide bond. The cell-penetrating moiety allows rapid and efficient transport through the plasma membrane. Once in the cytosol, the cell-penetrating moiety is released, thereby allowing C3 transferase to freely diffuse intracellularly and inactive RhoA, RhoB, and RhoC but not related GTPases such as Cdc42 or Rac1. C3 transferase inhibits Rho proteins by ADP ribosylation on Asn41 in the effector binding domain of the GTPase. Cells are then lysed and RhoA activity is measured by the RhoA G-LISA Activation Assay or alternatively cells are stained for F-actin[1]. |
| Cell Assay | Cell[1]The Narciclasine IC50 concentration, the Narciclasine concentration that decreased by 50% the global growth rate of a given cell population, is assessed with the MTT assay. The cells are incubated for 72 h in the presence and absence of the Narciclasine (with concentrations ranging between 10-9 and 10-5 M concentrate) for the determination of Narciclasine IC50 values[1]. |
| Animal Admin | Mice[1] The Hs683 cell line and GL19 primoculture grafted into the brains of nude immunodeficient mice both produced invasive brain tumors. Xenograft-bearing mice receive vehicle alone, oral temozolomide at 40 mg/kg (5 administrations per week for 5 consecutive weeks), or Narciclasine at 1 mg/kg either oral (once per week for 5 weeks) or i.v. (twice per week for 5 weeks). Drug administration is initiated respectively on days 5 and 7 post-tumor grafting for the Hs683 and GL19 models. The temozolomide dose and treatment schedule are selected based on previous optimized regimens. Narciclasine dose and treatment schedule are selected based on Narciclasine toxicity study in rats after oral administration and pharmacokinetic study that we have recently published. In toxicity study, Narciclasine (25, 10, or 1 mg/kg) is administered five times a week for 3 weeks and the no adverse effect level dose is defined to be 1 mg/kg/d p.o., with minimal acanthosis reactive changes and minor variations in some biochemistry parameters observed at this dose level considered to be nonadverse. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 733.8±60.0 °C at 760 mmHg |
| Molecular Formula | C14H13NO7 |
| Molecular Weight | 307.255 |
| Flash Point | 397.6±32.9 °C |
| Exact Mass | 307.069214 |
| PSA | 128.48000 |
| LogP | 0.59 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.797 |
| InChIKey | LZAZURSABQIKGB-AEKGRLRDSA-N |
| SMILES | O=C1NC2C(=CC(O)C(O)C2O)c2cc3c(c(O)c21)OCO3 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H340 |
| Precautionary Statements | P201-P308 + P313 |
| RIDADR | NONH for all modes of transport |
|
Simultaneous quantification of Amaryllidaceae alkaloids from Zephyranthes grandiflora by UPLC-DAD/ESI-MS/MS.
J. Pharm. Biomed. Anal. 71 , 187-92, (2012) A rapid, simple and sensitive ultra performance liquid chromatography-diode array detection method (UPLC-DAD) was developed and validated for quantification of four biologically important Amaryllidace... |
|
|
Seasonal accumulation of major alkaloids in organs of pharmaceutical crop Narcissus Carlton.
Phytochemistry 88 , 43-53, (2013) Narcissus pseudonarcissus (L.) cv. Carlton is being cultivated as a main source of galanthamine from the bulbs. After galanthamine, haemanthamine and narciclasine are the next most abundant alkaloids ... |
|
|
[Study on chemical constituents of the bulbs of Lycoris longituba].
Zhong Yao Cai 34(9) , 1366-8, (2011) To study the chemical constituents of the Bulbs of Lycoris longituba.The chemical constituents were isolated and purified by means of chromatographic techniques and their structures were elucidated by... |
| Lycoricidinol |
| Lycoricidin-A |
| (2S,3R,4S,4aR)-2,3,4,7-tetrahydroxy-3,4,4a,5-tetrahydro-2H-[1,3]dioxolo[4,5-j]phenanthridin-6-one |
| (2S,3R,4S,4aR)-2,3,4,7-Tetrahydroxy-3,4,4a,5-tetrahydro[1,3]dioxolo[4,5-j]phenanthridin-6(2H)-one |
| [1,3]Dioxolo[4,5-j]phenanthridin-6(2H)-one, 3,4,4a,5-tetrahydro-2,3,4,7-tetrahydroxy-, (2S,3R,4S,4aR)- |
| Narciclasine |
 CAS#:54162-19-5
CAS#:54162-19-5 CAS#:82299-36-3
CAS#:82299-36-3![(+)-methyl (1β,2α,6β)-2-hydroxy-7-oxabicyclo[4,1,0]hept-3-ene-4-carboxylate Structure](https://image.chemsrc.com/caspic/051/106861-61-4.png) CAS#:106861-61-4
CAS#:106861-61-4![5-bromo-4-(1-ethoxyethoxy)-2H-benzo[d]1,3-dioxolane Structure](https://image.chemsrc.com/caspic/228/192210-65-4.png) CAS#:192210-65-4
CAS#:192210-65-4![methyl 5-(butyryloxy)-7-oxabicyclo[4.1.0]hept-3-ene-3-carboxylate Structure](https://image.chemsrc.com/caspic/091/288395-83-5.png) CAS#:288395-83-5
CAS#:288395-83-5
