Eledoisin-Related Peptide TFA
Modify Date: 2024-01-05 22:49:52
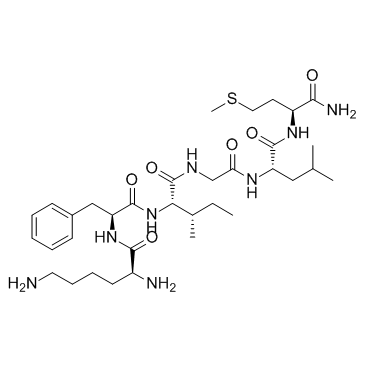
Eledoisin-Related Peptide TFA structure
|
Common Name | Eledoisin-Related Peptide TFA | ||
|---|---|---|---|---|
| CAS Number | 2990-43-4 | Molecular Weight | 706.93900 | |
| Density | 1.166g/cm3 | Boiling Point | 1083.4ºC at 760mmHg | |
| Molecular Formula | C34H58N8O6S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 609ºC | |
Use of Eledoisin-Related Peptide TFAEledoisin Related Peptide is a Substance P analog that excites neurons and triggers behavioral responses. Eledoisin Related Peptide is also a tachykinin receptor ligand. |
| Name | (2S)-2,6-diamino-N-[(2S)-1-[[(2S,3S)-1-[[2-[[(2S)-1-[[(2S)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]hexanamide |
|---|---|
| Synonym | More Synonyms |
| Description | Eledoisin Related Peptide is a Substance P analog that excites neurons and triggers behavioral responses. Eledoisin Related Peptide is also a tachykinin receptor ligand. |
|---|---|
| Related Catalog | |
| Target |
Tachykinin receptor[1] |
| In Vivo | Eledoisin Related Peptide shares with Substance P (SP) a common N-terminal amino acid sequence and has been shown by to have SP-like activity in the periphery (gut and salivary glands) and the CNS. Eledoisin-related peptide seems to be roughly equipotent with Substance P at identical ejection currents on the single-cell activity of neurons in this nucleus[2]. Both glutamate and substance P (and its analogue, eledoisin-related peptide) have excitatory effects on the activity of respiratory neurons and reflex interneurons[3]. |
| Animal Admin | Rats[2] Twenty-two male albino rats (230-260 g) are anesthetized with chloral hydrate (400 mg/kg, i.p.) and prepared for recording. Briefly, a singlebarrel recording pipette (tip 1 μm) is glued alongside a conventional five-barrel micropipette (tip 15-25/μm) then filled with 2 M NaCI saturated with Fast Green (impedance 4-7 M). The distance between the tip of the recording electrode and that of the five-barrel micropipette is 15-25/zm. Fast Green is ejected at the end of the experiment to identify the recording site. One side barrel of the five-barrel micropipette is loaded with 4 M NaCI for automatic current balancing and the others with three of the following solutions: L-epinephrine bitartrate (0. l M, pH 4.0), L-norepinephrine bitartrate (0.1 M, pH 4.0), Substance P (2.75 mM), physalaemin (2.6 mM), substance P 4-11 octapeptide (3.1 mM), eledoisin-related peptide (20 mM), neurotensin, bradykinin triacetate (15 mM), met-enkephalin (6.5 mM), TRH (48 mM). Spontaneously active cells are recorded in the locus coeruleus or in the nearby mesencephalic nucleus of the fifth nerve whose cells are easily identified by their increased activity upon manipulation of the jaw[2]. |
| References |
[1]. Severini C, et al. The tachykinin peptide family. Pharmacol Rev. 2002 Jun;54(2):285-322. |
| Density | 1.166g/cm3 |
|---|---|
| Boiling Point | 1083.4ºC at 760mmHg |
| Molecular Formula | C34H58N8O6S |
| Molecular Weight | 706.93900 |
| Flash Point | 609ºC |
| Exact Mass | 706.42000 |
| PSA | 265.93000 |
| LogP | 4.12680 |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.549 |
| Storage condition | 2-8℃ |
| Precursor 0 | |
|---|---|
| DownStream 4 | |
| Lysyl-phenylalanyl-isoleucyl-glycyl-leucyl-methioninamide |
| Lys-Phe-Ile-Gly-Leu-MetNH2 |
| Lpiglm |
| Lys-phe-ile-gly-leu-met-amide |
| L-Methioninamide,L-lysyl-L-phenylalanyl-L-isoleucylglycyl-L-leucyl |
| LYS-PHE-ILE-GLY-LEU-MET-NH2 |
| Eledoisin Related Peptide |
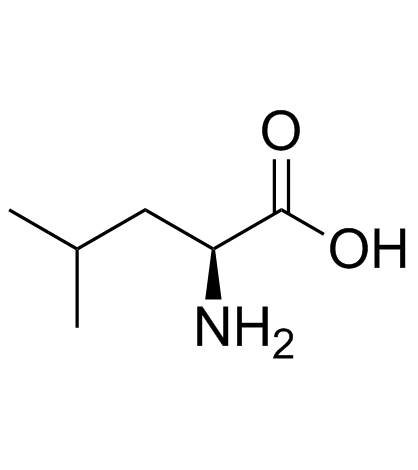 CAS#:61-90-5
CAS#:61-90-5 CAS#:73-32-5
CAS#:73-32-5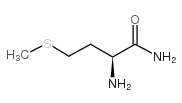 CAS#:4510-08-1
CAS#:4510-08-1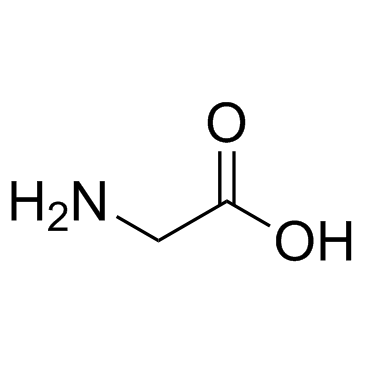 CAS#:56-40-6
CAS#:56-40-6
