O-Tritylhydroxylamine
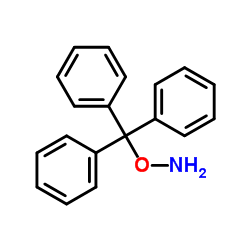
O-Tritylhydroxylamine structure
|
Common Name | O-Tritylhydroxylamine | ||
|---|---|---|---|---|
| CAS Number | 31938-11-1 | Molecular Weight | 275.344 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 443.2±44.0 °C at 760 mmHg | |
| Molecular Formula | C19H17NO | Melting Point | 79-81ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 235.1±22.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | o-tritylhydroxylamine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 443.2±44.0 °C at 760 mmHg |
| Melting Point | 79-81ºC(lit.) |
| Molecular Formula | C19H17NO |
| Molecular Weight | 275.344 |
| Flash Point | 235.1±22.1 °C |
| Exact Mass | 275.131012 |
| PSA | 35.25000 |
| LogP | 5.15 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.616 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922199090 |
|
~91% 
O-Tritylhydroxy... CAS#:31938-11-1 |
| Literature: Bella, A. Franco; Slawin, Alexandra M. Z.; Walton, John C. Journal of Organic Chemistry, 2004 , vol. 69, # 18 p. 5926 - 5933 |
|
~% 
O-Tritylhydroxy... CAS#:31938-11-1 |
| Literature: Journal of Organic Chemistry, , vol. 69, # 18 p. 5926 - 5933 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Multi-target spectral moment QSAR versus ANN for antiparasitic drugs against different parasite species.
Bioorg. Med. Chem. 18 , 2225-31, (2010) There are many of pathogen parasite species with different susceptibility profile to antiparasitic drugs. Unfortunately, almost QSAR models predict the biological activity of drugs against only one pa... |
|
|
Antimalarial and antileishmanial activities of histone deacetylase inhibitors with triazole-linked cap group.
Bioorg. Med. Chem. 18(1) , 415-25, (2010) Histone deacetylase inhibitors (HDACi) are endowed with plethora of biological functions including anti-proliferative, anti-inflammatory, anti-parasitic, and cognition-enhancing activities. Parsing th... |
|
|
Design and synthesis of novel histone deacetylase inhibitor derived from nuclear localization signal peptide
Bioorg. Med. Chem. Lett. 19(23) , 6588-90, (2009) We describe herein the synthesis and characterization of a new class of histone deacetylase (HDAC) inhibitors derived from conjugation of a suberoylanilide hydroxamic acid-like aliphatic-hydroxamate p... |
| O-triphenylmethyloxyamine |
| O-(Triphenylmethyl)hydroxylamine |
| EINECS 250-868-1 |
| O-Tritylhydroxylamine |
| triphenylmethoxyamine |
| Trityloxyamine |
| MFCD00042818 |
| Hydroxylamine, O-(triphenylmethyl)- |
| Trityl-O-Hydroxylamine |
| NH2-O-trityl |
| 1,1',1''-[(Aminooxy)methanetriyl]tribenzene |
 CAS#:10229-67-1
CAS#:10229-67-1
